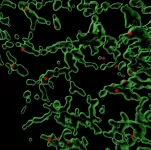
(Press-News.org) Scientists have created a new treatment for traumatic brain injury (TBI) that shrank brain lesions by 56% and significantly reduced local inflammation levels in pigs. The new approach leverages macrophages, a type of white blood cell that can dial inflammation up or down in the body in response to infection and injury. The team created disc-shaped microparticles called “backpacks” containing anti-inflammatory molecules, then attached them directly to the macrophages. These molecules kept the cells in an anti-inflammatory state when they arrived at the injury site in the brain, enabling them to reduce local inflammation and mitigate the damage caused. The research is reported in PNAS Nexus.
“Every year, millions of people suffer from a TBI, but there is currently no treatment beyond managing symptoms. We have applied our cellular backpack technology - which we previously used to improve macrophages’ inflammatory response to cancerous tumors - to deliver localized anti-inflammatory treatment in the brain, which helps mitigate the cascade of runaway inflammation that causes tissue damage and death in a human-relevant model,” said senior author Samir Mitragotri, Ph.D., in whose lab the research was performed. Mitragotri is a Core Faculty member of the Wyss Institute at Harvard University and the Hiller Professor of Bioengineering and Hansjörg Wyss Professor of Biologically Inspired Engineering at Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS).
Stopping a runaway inflammation train
More than a million people in the US suffer from a traumatic brain injury (TBI) every year, about 230,000 of them are hospitalized, and almost 70,000 die from TBI-related causes. There is currently no treatment for the damage caused to brain tissue during a TBI, beyond managing a patient’s symptoms. One of the main drivers of TBI-caused damage is a runaway inflammatory cascade in the brain.
As cells die from the impact, they release a cocktail of pro-inflammatory cytokine molecules that attract immune cells to clean up the damage. But the same cytokine molecules can also disrupt the blood-brain barrier, which causes blood to leak into the brain. Blood accumulation in the brain causes swelling, impaired oxygen delivery, and increased inflammation, and creates a vicious cycle of bleeding and damage that drives even more cell death.
The Mitragotri lab saw an opportunity in this problem.
“It’s generally believed anti-inflammatory therapies can be effective for treating TBI, but so far, none of them have proven effective clinically. Our previous work with macrophages has shown us that we can use our backpack technology to effectively steer their behavior when they arrive at the injury site. Since these cells are already active players in the body’s natural immune response to a TBI, we had a hunch we could augment that preexisting biology to reduce the initial damage,” said co-first author Rick Liao, Ph.D., a Postdoctoral Fellow at the Wyss Institute and SEAS.
“Body, heal thyself”…with backpacks
Macrophages are very malleable cells and can “switch” between pro-inflammatory and anti-inflammatory states. While the team’s previous work in cancer had been focused on keeping macrophages in a pro-inflammatory state when they arrive at the inflammation-reducing microenvironment of a tumor, this new project would be trying to do the opposite: keep the macrophages “calm” in the inflammation-riddled setting of a brain injury.
To do so, they used a disc-shaped “backpack” they had previously designed to treat multiple sclerosis that contained layers of two anti-inflammatory molecules: dexamethasone, a steroid, and interleukin-4, a cytokine that encourages macrophages to adopt an anti-inflammatory state. They then incubated these microparticles with both human and pig macrophages in vitro and saw that the backpacks stably stuck to the cells without causing any negative effect. They also observed that application of their backpacks decreased the expression of pro-inflammatory biomarkers and increased the expression of anti-inflammatory biomarkers, retaining the pig macrophages in a healing state.
But to prove that this shift would work in the body, they had to test the backpack-bearing macrophages in vivo. They chose pigs as their model organism because their brains’ structures and responses to injury more closely mimic those of humans than mice.
“Probably our biggest challenge in this project was scaling up production to match what we needed to run the experiments. Our previous studies were done in rodents, which required about two million macrophages and four million backpacks administered per subject. For the porcine study, we needed 100 million macrophages and 200 million backpacks per subject - on the scale of what would be administered in humans - and lots of helping hands,” said co-first author Neha Kapate, Ph.D., a Postdoctoral Fellow at the Wyss Institute and SEAS. The final team consisted of over 20 members from across the Wyss Institute, Harvard, MIT, and Mass General Hospital (MGH).
Once they had generated enough backpack-wearing porcine macrophages, they infused them into the pigs’ bloodstreams four hours after a TBI. Seven days later, they analyzed the animals’ brains. Pigs that had received the macrophage treatment showed a high concentration of the cells in the area immediately surrounding the injury site, their lesions were 56% smaller, and there was significantly less hemorrhaging than in untreated animals.
Local immune cells also displayed a lower amount of a pro-inflammatory activation marker called CD80, indicating that the macrophages had accomplished their damage control by reducing inflammation in the brain. Corroborating that data, the levels of two soluble biomarkers for inflammation in the blood and cerebrospinal fluid were lower in treated animals than in untreated animals. The macrophage treatment also did not cause any negative effects.
The team plans to conduct future studies that focus on elucidating exactly how their anti-inflammatory macrophage therapy affects the blood-brain barrier’s integrity to prevent bleeding, which could also hold promise for treating other conditions like hemorrhagic strokes.
“Macrophages’ susceptibility to their local environment has historically prevented scientists from taking full advantage of their immune-modulating capabilities. This impressive study describes a truly novel and potentially powerful macrophage-based therapy for treating the inflammation that is the root cause of so many human afflictions in an effective and non-invasive way that works with biology rather than against it,” said Wyss Founding Director Donald Ingber, M.D., Ph.D. Ingber is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and the Hansjörg Wyss Professor of Bioinspired Engineering at SEAS.
Additional authors of the study include Luke Sodemann, Tawny Stinson, Bryan Golemb, Alexander Hone, and Andrea Slate from MGH; Supriya Prakash, Ninad Kumbhojkar, Vineeth Chandran Suja, Suyog Shaha, Kyung Soo Park, Michael Dunne, and Kolade Adebowale from the Wyss Institute and SEAS; Lily Li-Wen Wang and Morgan James from the Wyss Institute, Harvard SEAS, and MIT; Mikayla Flanz, Rohan Rajeev, Dania Villafuerte, and John Clegg from Harvard SEAS; Declan McGuone from Yale School of Medicine, and Beth Costine-Bartell from MGH and Harvard Medical School.
This research was supported by the US Department of Defense under Grant No. W81XWH-19-2-0011, the National Science Foundation Graduate Research Fellowship under Grant Nos. 1122374 and 1745302, National Institute of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development under Grant No. R01 HD099397, the Wyss Institute at Harvard University, and Harvard SEAS.
END
Using the body’s own cells to treat traumatic brain injury
Macrophages bearing microparticle “backpacks” reduce lesion size and inflammation at the site of brain injuries, mitigating damage
2024-01-03
ELSE PRESS RELEASES FROM THIS DATE:
UW–Madison scientists reveal the inner workings of an essential protein trafficking complex
2024-01-03
MADISON – Like mail carriers who manage to deliver their parcels through snow, rain, heat and gloom, a critical group of mammalian proteins helps cells function properly even under less-than-ideal conditions.
Using state-of-the-art cell imaging and genome editing technology, University of Wisconsin–Madison scientists have begun to unravel how this collection of proteins performs its essential service. The discovery could eventually help researchers better understand and develop new treatments for diseases like cancer, diabetes and those that cause immune dysfunction.
Led by Anjon Audhya, a professor in the Department of Biomolecular Chemistry, the research team sought ...
Mapping of the gene network that regulates glycan clock of ageing
2024-01-03
“[...] we were able to confirm the functional role of three genes (MANBA, TNFRSF13B and EEF1A1) in the IgG galactosylation pathway [...]”
BUFFALO, NY- January 3, 2024 – A new research paper was published in Aging (listed by MEDLINE/PubMed as "Aging (Albany NY)" and "Aging-US" by Web of Science) Volume 15, Issue 24, entitled, “Mapping of the gene network that regulates glycan clock of ageing.”
Glycans are an essential structural component of immunoglobulin G (IgG) that modulate its structure and function. However, regulatory mechanisms behind this complex posttranslational ...
Computational method discovers hundreds of new ceramics for extreme environments
2024-01-03
DURHAM, N.C. – If you have a deep-seated, nagging worry over dropping your phone in molten lava, you’re in luck.
A research team led by materials scientists at Duke University has developed a method for rapidly discovering a new class of materials with heat and electronic tolerances so rugged that they that could enable devices to function at lava-like temperatures above several thousands of degrees Fahrenheit.
Harder than steel and stable in chemically corrosive environments, these materials ...
Community cancer care linked with poorer outcomes for patients with a common head and neck cancer
2024-01-03
FOR IMMEDIATE RELEASE
Care for patients with human papillomavirus (HPV)-related squamous cell cancers of the oropharynx (an area in back of the throat) is shifting toward community cancer centers, but patients treated in this setting may be less likely to survive, according to new research by investigators from the Johns Hopkins Kimmel Cancer Center and its Head and Neck Cancer Center.
The study, published Jan. 3 in the Journal of the National Cancer Institute, raises concerns about the quality of care that patients with this type of head and neck cancer receive outside of academic medical centers. Patients treated ...
Beta blocker used to treat heart problems and other medical concerns could be new treatment for sickle cell cardiomyopathy
2024-01-03
INDIANAPOLIS—A beta blocker typically used to treat heart problems, hemangioma, migraines and anxiety could be a new therapeutic for patients with sickle cell disease. Researchers led by Ankit A. Desai, MD, associate professor of medicine at the Krannert Cardiovascular Research Center (KCVRC) at Indiana University School of Medicine, have been awarded a $3 million grant by the U.S. Department of Defense to evaluate the efficacy of this drug.
Patients with sickle cell disease, a red blood ...
Cleveland Clinic and University of Western Ontario awarded $4.9 million from the Helmsley Charitable Trust to build Crohn’s disease and ileostomy research consortium
2024-01-03
Cleveland Clinic and the University of Western Ontario have been awarded a $4.9 million grant from Helmsley Charitable Trust to build a consortium to develop clinical trial outcome tools for patients with Crohn's disease and permanent ileostomy.
A permanent ileostomy is a surgical procedure to divert stool from the intestine after removing part of or the whole colon. The new Endpoint Development for Ostomy Clinical Trial (EndO-trial) Consortium seeks to develop more effective drugs for patients with Crohn's disease who have undergone ...
Researchers improve seed nitrogen content by reducing plant chlorophyll levels
2024-01-03
Chlorophyll plays a pivotal role in photosynthesis, which is why plants have evolved to have high chlorophyll levels in their leaves. However, making this pigment is expensive because plants invest a significant portion of the available nitrogen in both chlorophyll and the special proteins that bind it. As a result, nitrogen is unavailable for other processes. In a new study, researchers reduced the chlorophyll levels in leaves to see if the plant would invest the nitrogen saved into other process that might improve nutritional quality.
Over the past few decades, researchers ...
How does corrosion happen? New research examines process on atomic level
2024-01-03
BINGHAMTON, N.Y. -- When water vapor meets metal, the resulting corrosion can lead to mechanical problems that harm a machine’s performance. Through a process called passivation, it also can form a thin inert layer that acts as a barrier against further deterioration.
Either way, the exact chemical reaction is not well understood on an atomic level, but that is changing thanks to a technique called environmental transmission electron microscopy (TEM), which allows researchers to directly view molecules interacting ...
Survival of the fittest: Words like 'Sex' and 'fight' are most likely to stand the test of time
2024-01-03
New research from the University of Warwick reveals that words like 'sex' endure in our language in a ‘survival of the fittest’ way, similar to natural selection.
Whilst the recent announcement of Word of the Year explores new words, like ‘rizz’ or ‘situationship’, Professor Thomas Hills’ research delves into why some words survive in our modern linguistic landscape, while others don’t.
The study concludes that words with the strongest lasting power are:
Words acquired earlier in life
Words associated with things people can see ...
NYU Grossman School of Medicine and Sarah Lawrence College launch graduate genomics degree program
2024-01-03
NYU Grossman School of Medicine’s Vilcek Institute of Graduate Biomedical Sciences and Sarah Lawrence College today announced the launch of a master’s program in genome health analysis (GHA).
Building on the strengths of both institutions, the new program will focus on analyses of patient genomes, the complete sets of genetic information in human cells. New tools have dramatically increased the amount and quality of genomic data available on each patient, but the field is constrained by the small number of experts trained to use and apply ...
LAST 30 PRESS RELEASES:
Scientists discover why we know when to stop scratching an itch
A hidden reason inner ear cells die – and what it means for preventing hearing loss
Researchers discover how tuberculosis bacteria use a “stealth” mechanism to evade the immune system
New microscopy technique lets scientists see cells in unprecedented detail and color
Sometimes less is more: Scientists rethink how to pack medicine into tiny delivery capsules
Scientists build low-cost microscope to study living cells in zero gravity
The Biophysical Journal names Denis V. Titov the 2025 Paper of the Year-Early Career Investigator awardee
Scientists show how your body senses cold—and why menthol feels cool
Scientists deliver new molecule for getting DNA into cells
Study reveals insights about brain regions linked to OCD, informing potential treatments
Does ocean saltiness influence El Niño?
2026 Young Investigators: ONR celebrates new talent tackling warfighter challenges
Genetics help explain who gets the ‘telltale tingle’ from music, art and literature
Many Americans misunderstand medical aid in dying laws
Researchers publish landmark infectious disease study in ‘Science’
New NSF award supports innovative role-playing game approach to strengthening research security in academia
Kumar named to ACMA Emerging Leaders Program for 2026
AI language models could transform aquatic environmental risk assessment
New isotope tools reveal hidden pathways reshaping the global nitrogen cycle
Study reveals how antibiotic structure controls removal from water using biochar
Why chronic pain lasts longer in women: Immune cells offer clues
Toxic exposure creates epigenetic disease risk over 20 generations
More time spent on social media linked to steroid use intentions among boys and men
New study suggests a “kick it while it’s down” approach to cancer treatment could improve cure rates
Milken Institute, Ann Theodore Foundation launch new grant to support clinical trial for potential sarcoidosis treatment
New strategies boost effectiveness of CAR-NK therapy against cancer
Study: Adolescent cannabis use linked to doubling risk of psychotic and bipolar disorders
Invisible harms: drug-related deaths spike after hurricanes and tropical storms
Adolescent cannabis use and risk of psychotic, bipolar, depressive, and anxiety disorders
Anxiety, depression, and care barriers in adults with intellectual and developmental disabilities
[Press-News.org] Using the body’s own cells to treat traumatic brain injuryMacrophages bearing microparticle “backpacks” reduce lesion size and inflammation at the site of brain injuries, mitigating damage