Monoclonal antibodies (mAbs) are laboratory-designed proteins that mimic the immune system's antibodies. To date, many therapeutic mAbs belonging to the immunoglobulin G (IgG) class of antibodies, have been approved for the treatment of cancer and autoimmune diseases. Cell lines such as the Chinese hamster ovary (CHO) cells are generally used to produce mAbs. Notably, the production and manufacture of mAbs are regulated by critical quality attributes (CQAs) to ensure their safety and efficacy in treatment.
An important CQA for mAbs is the N-linked glycosylation present at a specific position (Asn297). N-linked glycans consist of N-acetylglucosamine (GlcNAc), mannose (Man), fucose (Fuc), galactose (Gal), and sialic acid. The heterogeneity of the N-linked glycan profiles of mAbs can be attributed to the different numbers and linkages of additional saccharides. The composition of N-linked glycans affects the overall therapeutic efficacy, targeting ability, and immune-specificity of these antibodies. For example, antibody-dependent cellular cytotoxicity (ADCC) is influenced by the fucosylation and galactosylation of N-linked glycans. Complement-dependent cytotoxicity (CDC) is also affected by the galactosylation and sialylation of N-linked glycans. Hence, it's crucial to meticulously regulate N-linked glycan profiles throughout the manufacturing process because the heterogeneity of the N-linked glycan profile of mAbs depends on the cell culture duration and changes in nucleotide sugars and glycosylation enzyme levels.
Recently, Dr. Kyohei Higashi, Associate Professor at Tokyo University of Science (TUS) in Japan, along with a team of researchers including Dr. Rin Miyajima and Dr. Masahiro Komeno, conducted a study to explore the effects of polyamines on N-linked glycan profiles of mAbs in CHO DP-12 cells. Their work was made available online on November 3, 2023 in the Journal of Biotechnology.
“Because the carbohydrate structure of mAbs changes depending on the state of the cells, we were interested in investigating the relationship between intracellular polyamines and the carbohydrate structure of mAbs from CHO cells.” explained Dr. Higashi, when asked about the motivation behind the research.
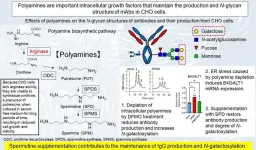
Polyamines (putrescine, PUT; spermidine, SPD; and spermine, SPM) are present in millimolar concentrations in all living organisms and play essential roles in normal cell growth and differentiation. PUT, SPD, and SPM contained two, three, and four amino groups, respectively. PUT is synthesized from ornithine (ORN) by ornithine decarboxylase (ODC), a rate-limiting enzyme in the polyamine biosynthesis pathway. SPD is synthesized from putrescine by spermidine synthase, and spermine is synthesized from spermidine by spermine synthase. Intracellular polyamine levels are regulated at various steps, including synthesis, degradation, and transport, and are affected by external stimuli, aging, and diseases. Because CHO cells lack arginase activity to produce ORN from arginine, they cannot produce polyamines in serum-free media, resulting in a decrease in intracellular polyamine levels, which causes a low growth rate and cell viability during long-term cultivation.
Intracellular polyamine levels can also be decreased by treatment with α-difluoromethylornithine (DFMO), an inhibitor of ODC. The depletion of intracellular polyamines by DFMO can be reversed by the addition of SPD to the growth media of CHO cells.
Upon introducing DFMO to the CHO cells, the team observed that IgG antibody galactosylation surged, along with an increase in the levels of β1,4-galactosyl transferase 1 (B4GALT1) mRNA. This mRNA is pivotal in governing the IgG galactosylation mechanism within CHO cells. What’s more, IgG production decreased by approximately 30% in DFMO-treated cells.
Dr. Higashi hypothesized that the decrease in IgG production was a result of endoplasmic stress (ER) stress response caused by polyamine depletion. During ER stress response, protein folding ceases, resulting in the arrest of the normal function of cells. Chaperone proteins assist in the correct folding of other protein classes and play a crucial role under both normal and stress conditions. The results of the ER stress response study confirmed the increased expression levels of chaperones for glycoprotein folding, in polyamine-depleted cells.
The team further observed that upon using tunicamycin, an ER stress inducer inhibiting N-glycosylation, ER stress from polyamine depletion triggered B4GALT1 mRNA expression, increasing IgG galactosylation in CHO cells.
The ability to maintain antibody glycosylation profiles via polyamine modulation has numerous implications. Controlled glycosylation is crucial for optimizing therapeutic proteins, such as antibodies, ensuring the stable production of antibodies in a uniform manner of biological activities, and potentially decreasing the manufacturing cost. Supplementation of polyamine could be accomplished by the addition only of SPD to serum-free medium, offer an easy and costless method to maintain the glycan structure of mAbs produced by CHO cells cultured in the serum-free medium. This insight might influence cell line development and bioproduction, facilitating the creation of biosimilars.
“Introducing polyamines, particularly SPD, to serum-free culture medium for CHO cells may contribute to consistent manufacturing and quality control of antibody production. We hope that this research will contribute to the stable production of antibody drugs and lead to lower drug prices” concludes Dr. Higashi.
***
Reference
DOI: https://doi.org/10.1016/j.jbiotec.2023.10.008
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Dr. Kyohei Higashi from Tokyo University of Science
Dr. Kyohei Higashi is an Associate Professor at the Department of Pharmacy, Faculty of Pharmaceutical Sciences, Tokyo University of Science, Japan. He completed his Ph.D. in pharmaceutical sciences from Chiba University, Japan. Over the last two decades, he has published 90 research articles, which have been cited more than 1,800 times. His research explores physical pharmacy and clinical and analytical biochemistry, with a focus on polyamines and glycosaminoglycans. He is also active in the research areas of molecular biology, cell biology, and molecular genetics. He recently received the Carbohydrate Research JSCR42 Poster Award.
Funding Information
This study was supported by a Grant-in-Aid for Scientific Research (C) (No. 18K06652) from the Japan Society for the Promotion of Science to Associate Professor Dr. Kyohei Higashi.
END