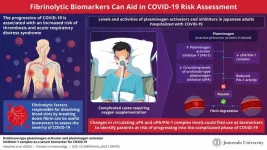
The global impact of the COVID-19 pandemic on healthcare systems has been significant. The sudden surge in infected cases overwhelmed hospitals and disrupted routine healthcare services, thus further worsening public health. Managing patients, too, has been challenging due to the variation of COVID-19 symptoms, ranging from mild to severe, that require medical intervention.
To help hospitals prioritize patients in need of care, researchers have been looking into various biological markers that can determine the risk of the disease becoming more severe. Among these, proteins in the blood related to blood clot formation, increased inflammation, and dysfunction of blood vessels have shown promise. One specific protein of interest is plasminogen, which, when activated to plasmin, dissolves blood clots by breaking down fibrin, which is the mesh-like structure holding the clot together. However, many individuals with COVID-19 tend to have lower levels of this critical protein.
Now, in a study published in the journal Frontiers in Immunology on 11 January 2024, researchers from Juntendo University in Japan led by Specially Appointed Senior Associate Professor Koichi Hattori, along with Associate Professor Beate Heissig and Japan Society for the Promotion of Science researcher Tetiana Yatsenko, find that proteins regulating plasminogen-plasmin levels can be used as biomarkers to identify individuals who are at risk of progressing to a severe stage of the disease. Japan Society for the Promotion of Science researcher Tetiana Yatsenko, find that proteins regulating plasminogen-plasmin levels can be used as biomarkers to identify individuals who are at risk of progressing to a severe stage of the disease.
“It is (often) difficult to judge the severity of a patient at diagnosis with conventional tests. But identifying high-risk patients, such as those who might require oxygen supplementation as early as possible, will save lives. We have discovered biomarkers to determine the risk of the disease becoming more severe at diagnosis,” says Dr. Hattori.
The researchers specifically looked at the levels of three different proteins associated with blood clot formation: urokinase-type plasminogen activator (uPA), tissue-type plasminogen activator (tPA), and plasminogen activator inhibitor-1 (PAI-1) in hospitalized Japanese adults with COVID-19.
uPA and tPA are proteins that activate plasminogen to plasmin. PAI-I inhibits the plasminogen activators by binding with them, forming uPA/PAI-1 and tPA/PAI-1 complexes. Patients requiring oxygen supplementation tend to have the highest levels of PAI-1 protein compared to those displaying mild symptoms and a healthy control group. However, the levels of active PAI-1 that restrict plasmin activation remained similar in all the groups.
Out of the two plasmin activators, uPA levels were lower in COVID-19 patients than in the healthy control group. Patients who required oxygen supplementation had the lowest uPA/PAI-1 complex levels. Conversely, tPA levels were similar in all groups. There were no differences in tPA/PAI-1 levels among patients displaying mild and severe symptoms.
The researchers found PAI-1 and uPA/PAI-1 complex-related changes in response to proinflammatory factors, which, in turn, were released in response to the viral infection. The researchers found the higher levels of PAI-1 to correlate with soluble urokinase plasminogen activator receptor (suPAR) and soluble vascular cell adhesion molecule-1 (sVCAM-1), two proteins released by endothelial cells in response to inflammation caused by the SARS-CoV-2 virus. This finding suggests that heightened PAI-1 levels can serve as a valuable indicator of endothelial dysfunction associated with COVID-19.
Acute respiratory distress syndrome (ARDS) is one of the most feared complications of COVID-19, a condition of acute lung failure. Notably, the researchers discovered robust correlations between these proteins and ARDS and lymphopenia, conditions observed in severe cases of COVID-19. They identified a positive correlation between PAI-1 levels and ARDS (higher levels associated with severe ARDS) and an inverse correlation with lymphocytes. Conversely, the researchers observed that the uPA/PAI-1 complex levels are negatively correlated with ARDS and positively correlated with lymphocytes.
“Significant decreases in circulating uPA and uPA/PAI-1 complex levels may be a novel biomarker of COVID-19 severity,” says Dr. Hattori, summarizing the findings. This study can lead to portable test kits, which enhance screening and ensure more targeted healthcare interventions. “By identifying individuals who are at risk of progressing to a severe stage of COVID-19, we can reduce the strain on medical facilities as we can reserve emergency beds for those who might experience worsening of the disease and provide medical care tailored to the specific condition of each patient,” says Dr. Hattori who, along with his team, has taken a significant step by applying for a patent based on the findings.
Reference
Authors
Tetiana Yatsenko 1,2, Ricardo Rios 3, Tatiane Nogueira 3, Satoshi Takahashi 4, Yoko Tabe1, Toshio Naito 1, Kazuhisa Takahashi 1,5, Koichi Hattori 6* and Beate Heissig 1
Title of original paper
Urokinase-type plasminogen activator and plasminogen activator inhibitor-1 complex as a serum biomarker for COVID-19
Journal
Frontiers in Immunology
DOI
10.3389/fimmu.2023.1299792
Affiliations
1 Department of Research Support Utilizing Bioresource Bank, Graduate School of Medicine, Juntendo University School of Medicine
2 Department of Enzymes Chemistry and Biochemistry, Palladin Institute of Biochemistry of the National Academy of Science of Ukraine
3 Institute of Computing, Federal University of Bahia
4 Division of Clinical Precision Research Platform, the Institute of Medical Science, the University of Tokyo
5 Department of Respiratory Medicine, Juntendo University School of Medicine
6 Center for Genome and Regenerative Medicine, Juntendo University
About Senior Associate Professor Koichi Hattori from Juntendo University
Dr. Koichi Hattori is a specially appointed senior associate professor at the Graduate School of Medicine, Center for Genome Regenerative Medicine, Juntendo University. He is also a counselor of the Japanese Society for Regenerative Medicine and the Japanese Society of Hematology. He is also a special care physician at the Department of Hematology/Oncology at the Institute of Medical Science, the University of Tokyo. His research interests encompass modeling inflammatory diseases, cancer, and tissue regeneration. He has published over 110 papers on these topics. Additionally, he holds patents related to treating inflammatory diseases and tissue regeneration.
The global impact of the COVID-19 pandemic on healthcare systems has been significant. The sudden surge in infected cases overwhelmed hospitals and disrupted routine healthcare services, thus further worsening public health. Managing patients, too, has been challenging due to the variation of COVID-19 symptoms, ranging from mild to severe, that require medical intervention.
To help hospitals prioritize patients in need of care, researchers have been looking into various biological markers that can determine the risk of the disease becoming more severe. Among these, proteins in the blood related to blood clot formation, increased inflammation, and dysfunction of blood vessels have shown promise. One specific protein of interest is plasminogen, which, when activated to plasmin, dissolves blood clots by breaking down fibrin, which is the mesh-like structure holding the clot together. However, many individuals with COVID-19 tend to have lower levels of this critical protein.
Now, in a study published in the journal Frontiers in Immunology on 11 January 2024, researchers from Juntendo University in Japan led by Specially Appointed Senior Associate Professor Koichi Hattori, along with Associate Professor Beate Heissig and Japan Society for the Promotion of Science researcher Tetiana Yatsenko, find that proteins regulating plasminogen-plasmin levels can be used as biomarkers to identify individuals who are at risk of progressing to a severe stage of the disease. Japan Society for the Promotion of Science researcher Tetiana Yatsenko, find that proteins regulating plasminogen-plasmin levels can be used as biomarkers to identify individuals who are at risk of progressing to a severe stage of the disease.
“It is (often) difficult to judge the severity of a patient at diagnosis with conventional tests. But identifying high-risk patients, such as those who might require oxygen supplementation as early as possible, will save lives. We have discovered biomarkers to determine the risk of the disease becoming more severe at diagnosis,” says Dr. Hattori.
The researchers specifically looked at the levels of three different proteins associated with blood clot formation: urokinase-type plasminogen activator (uPA), tissue-type plasminogen activator (tPA), and plasminogen activator inhibitor-1 (PAI-1) in hospitalized Japanese adults with COVID-19.
uPA and tPA are proteins that activate plasminogen to plasmin. PAI-I inhibits the plasminogen activators by binding with them, forming uPA/PAI-1 and tPA/PAI-1 complexes. Patients requiring oxygen supplementation tend to have the highest levels of PAI-1 protein compared to those displaying mild symptoms and a healthy control group. However, the levels of active PAI-1 that restrict plasmin activation remained similar in all the groups.
Out of the two plasmin activators, uPA levels were lower in COVID-19 patients than in the healthy control group. Patients who required oxygen supplementation had the lowest uPA/PAI-1 complex levels. Conversely, tPA levels were similar in all groups. There were no differences in tPA/PAI-1 levels among patients displaying mild and severe symptoms.
The researchers found PAI-1 and uPA/PAI-1 complex-related changes in response to proinflammatory factors, which, in turn, were released in response to the viral infection. The researchers found the higher levels of PAI-1 to correlate with soluble urokinase plasminogen activator receptor (suPAR) and soluble vascular cell adhesion molecule-1 (sVCAM-1), two proteins released by endothelial cells in response to inflammation caused by the SARS-CoV-2 virus. This finding suggests that heightened PAI-1 levels can serve as a valuable indicator of endothelial dysfunction associated with COVID-19.
Acute respiratory distress syndrome (ARDS) is one of the most feared complications of COVID-19, a condition of acute lung failure. Notably, the researchers discovered robust correlations between these proteins and ARDS and lymphopenia, conditions observed in severe cases of COVID-19. They identified a positive correlation between PAI-1 levels and ARDS (higher levels associated with severe ARDS) and an inverse correlation with lymphocytes. Conversely, the researchers observed that the uPA/PAI-1 complex levels are negatively correlated with ARDS and positively correlated with lymphocytes.
“Significant decreases in circulating uPA and uPA/PAI-1 complex levels may be a novel biomarker of COVID-19 severity,” says Dr. Hattori, summarizing the findings. This study can lead to portable test kits, which enhance screening and ensure more targeted healthcare interventions. “By identifying individuals who are at risk of progressing to a severe stage of COVID-19, we can reduce the strain on medical facilities as we can reserve emergency beds for those who might experience worsening of the disease and provide medical care tailored to the specific condition of each patient,” says Dr. Hattori who, along with his team, has taken a significant step by applying for a patent based on the findings.
Reference
Authors
Tetiana Yatsenko 1,2, Ricardo Rios 3, Tatiane Nogueira 3, Satoshi Takahashi 4, Yoko Tabe1, Toshio Naito 1, Kazuhisa Takahashi 1,5, Koichi Hattori 6* and Beate Heissig 1
Title of original paper
Urokinase-type plasminogen activator and plasminogen activator inhibitor-1 complex as a serum biomarker for COVID-19
Journal
Frontiers in Immunology
DOI
10.3389/fimmu.2023.1299792
Affiliations
1 Department of Research Support Utilizing Bioresource Bank, Graduate School of Medicine, Juntendo University School of Medicine
2 Department of Enzymes Chemistry and Biochemistry, Palladin Institute of Biochemistry of the National Academy of Science of Ukraine
3 Institute of Computing, Federal University of Bahia
4 Division of Clinical Precision Research Platform, the Institute of Medical Science, the University of Tokyo
5 Department of Respiratory Medicine, Juntendo University School of Medicine
6 Center for Genome and Regenerative Medicine, Juntendo University
About Senior Associate Professor Koichi Hattori from Juntendo University
Dr. Koichi Hattori is a specially appointed senior associate professor at the Graduate School of Medicine, Center for Genome Regenerative Medicine, Juntendo University. He is also a counselor of the Japanese Society for Regenerative Medicine and the Japanese Society of Hematology. He is also a special care physician at the Department of Hematology/Oncology at the Institute of Medical Science, the University of Tokyo. His research interests encompass modeling inflammatory diseases, cancer, and tissue regeneration. He has published over 110 papers on these topics. Additionally, he holds patents related to treating inflammatory diseases and tissue regeneration.
END