Under strict embargo: 18:00hrs GMT Friday 22 March 2024
Peer reviewed
Experimental study
Animals
Researchers uncover protein interactions controlling fertility in female mice
Researchers at the Francis Crick Institute have shed light on the proteins controlling the development of ovaries in mice before and after birth. This could lead to a better understanding of how female infertility develops.
Following their research identifying the gene responsible for initiating the development of ovaries in the mouse embryo, the scientists aimed to understand which genes maintain the functions of the ovaries, including producing eggs, after birth.
Previous experiments have shown that removing a gene called Foxl2 in female (XX) mice at different points in development has different effects depending on the timing. If removed from embryos, ovaries become abnormal and the adult mice are infertile. If removed from adult mice, their ovaries begin to resemble testes1.
In research published today in Science Advances, the team found that, while FOXL2 does play a role during embryonic development, it has the most impact after birth, where the protein regulates the activity of many more genes, including some involved in functions critical for the ovary such as egg development.
FOXL2 is a type of protein that physically sits on top of specific regions in DNA (‘enhancers’) and influences whether and how other (target) genes are read.
The researchers used a technique called chromatin proteomics to ‘fish out’ all of the other proteins that interact with FOXL2 when it is bound to DNA. They found that the number of protein interactions drastically increased in ovaries after birth compared to during embryonic development.
Among many others, they identified a protein called USP7, which binds to FOXL2 when it interacts with its DNA targets. Until now, researchers weren’t aware of USP7 and FOXL2 interaction or what role USP7 was playing in ovary development.
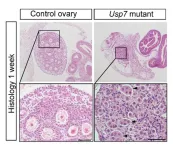
When the researchers removed the Usp7 gene from female mice, they found that the mice couldn’t develop ovaries beyond puberty, so were infertile. The team believe USP7 might be needed to stabilise FOXL2 on top of DNA.
FOXL2 and USP7 share some common roles in humans. People lacking one copy of the FOXL2 gene can start making eggs but don’t develop full ovaries, so have problems with fertility. USP7 mutations can also lead to infertility in people, as well as neurodevelopmental disorders.
Genetic testing is key to diagnose problems with sexual development, so researchers hope to find the major genetic causes of infertility and consider how gene editing techniques could help with future treatments.
Robin Lovell-Badge, Group Leader of the Stem Cell Biology and Developmental Genetics Laboratory at the Crick, said: “In our research, we’ve come closer to answers for two major questions regarding development – what drives ovary development, and how the function of the ovary is maintained. We’ve found that FOXL2 has very different roles throughout development, and identified another crucial protein, USP7.
“The genetic factors underlying female development haven’t been as well studied as male development, because many female developmental pathways happen at the same time rather than in an easy-to-follow sequence. Infertility is a big problem worldwide, so shedding light on the key genes and proteins responsible at each stage is vital.”
Roberta Migale, Postdoctoral Fellow at the Crick and first and co-senior author on the study, said: “This is the first time we’ve been able to use these approaches to see the interactions that FOXL2, a factor critical for female fertility, establishes with other proteins whilst they are bound to DNA in mouse ovaries. Factors that actively bind to the DNA are more likely to have an impact on the regulation of genes important for the development and function of the ovary. We’ve identified USP7 through this method and the hope is that many more proteins responsible for ovary development can be found using our approach.”
A Crick-wide effort, Robin and Roberta worked with several specialist teams, including the Genetic Modification Service, Bioinformatics and Biostatistics, Proteomics, Flow Cytometry, Experimental Histopathology, Light Microscopy, and the Biological Research Facility.
The researchers will continue to study the role of the USP7 protein in sexual development.
-ENDS-
For further information, contact: press@crick.ac.uk or +44 (0)20 3796 5252
Notes to Editors
Reference: Migale, R. et al. (2024). FOXL2 interaction with different binding partners regulates the dynamics of ovarian development. Science Advances. 10.1126/sciadv.adl0788.
(1) It has previously been shown that if a gene called Foxl2 is lacking in XX (female) mouse embryos, ovaries develop but become abnormal from a week after birth. The ‘granulosa’ cells that normally support the eggs within ovarian follicles (small spheres that become filled with fluid as eggs grow) fail to mature, which then affects the egg cells and the mice are infertile.
If Foxl2 is deleted instead from adult ovaries, the granulosa cells rapidly turn into Sertoli-like cells, and the gonads come to resemble testes. Sertoli cells are the testicular cells that usually support sperm formation in XY males, but the gonadal sex reversal seen in the adult Foxl2-deleted mice implies that they are also required to instruct other testis cell types to be present.
These experiments suggested that the FOXL2 protein has very different functions depending on the time of development, where its role in the embryo may be compensated by the action of other proteins, but after birth, it becomes the sole factor required to maintain ovaries.
The Francis Crick Institute is a biomedical discovery institute dedicated to understanding the fundamental biology underlying health and disease. Its work is helping to understand why disease develops and to translate discoveries into new ways to prevent, diagnose and treat illnesses such as cancer, heart disease, stroke, infections, and neurodegenerative diseases.
An independent organisation, its founding partners are the Medical Research Council (MRC), Cancer Research UK, Wellcome, UCL (University College London), Imperial College London and King’s College London.
The Crick was formed in 2015, and in 2016 it moved into a brand new state-of-the-art building in central London which brings together 1500 scientists and support staff working collaboratively across disciplines, making it the biggest biomedical research facility under a single roof in Europe.
http://crick.ac.uk/
END