Optoelectronic devices have found their way into many aspects of our daily lives, from OLED displays to photodetectors, security systems, and environmental monitoring. In all the applications, these devices utilize high refractive index polymers (HRIPs) to control light.
In general, the optical properties of transparent HRIPs enable efficient light transmission and manipulation, allowing optoelectronics devices to guide and control the flow of light to improve their performance. However, there are no low-cost options for HRIPs that can guarantee good optical performance while being transparent and environmentally friendly. This is because, for most materials, there exists an inherent trade-off between their refractive index, transparency, and processability.
Now, in a recent study, a research team led by Professor Kenichi Oyaizu from the Department of Applied Chemistry at Waseda University, Japan, found a way to circumvent this problem. In their article published in Advanced Functional Materials on April 12, 2024, the researchers report a novel type of aromatic HRIP whose properties make it a perfect candidate for modern optoelectronic applications. This article was co-authored by Seigo Watanabe from the Research Institute of Science and Engineering, Waseda University, as well as Luca M. Cavinato and Rubén D. Costa, both from the Chair of Biogenic Functional Materials, Technical University of Munich, Germany.
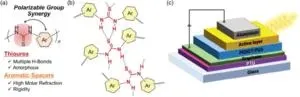
The proposed family of compounds are called poly(thiourea)s (PTUs), with each repeating unit of the polymer (the monomer) comprising a simple aromatic ring linked to a thiourea group (H2N−C(=S)−NH2). These PTUs have an exceptional property: the thiourea units in different polymer strands attract each other via hydrogen bonds, which is a type of intermolecular interaction. Simply put, the sulfur (S) atoms of a thiourea group attract the hydrogen (H) atoms linked to nitrogen (N) in another thiourea group due to local differences in electric charge.
These so-called ‘polarizable hydrogen bonds’ cause the PTU material to be densely packed, creating dense networks. Since the polymer is amorphous and has no crystalline order, it is highly transparent. Meanwhile, the aromatic rings serve as spacers, providing some rigidity and mechanical strength and contributing to a higher refractive index. The research team carefully analyzed the properties of these PTUs and demonstrated their potential by incorporating them into experimental optoelectronic components, obtaining remarkable results. More specifically, the proposed PTUs demonstrated a high transparency of over 92% and an exceptional refractive index of 1.81.
Notably, the team also investigated whether PTUs could be easily degraded into simpler useful molecules. “Due to recent environmental issues caused by plastic wastes, the degradation of polymers to monomers becomes an essential functionality leading to sustainable recycling. To the best of our knowledge, there have been extremely few attempts to impart degradability to HRIPs, and systematic designs for degradable HRIPs have not been reported despite such global needs,” comments Prof. Oyaizu. Their efforts led to a simple degradation protocol that involves mild heating conditions and mixing with diamines, which is enough to break up PTUs into smaller pieces that can be reprocessed or repurposed for the chemical synthesis of new PTUs.
Overall, the results of this study are very promising for the future of optoelectronic materials and devices in the greater context of sustainability. “Based on these findings, environmentally friendly optical materials would be easily preparable with a simple process, enabling sustainable optoelectronics such as low-cost bright displays, wearable lighting devices, and thinner, lighter, and degradable polymer eyeglasses,” concludes Prof. Oyaizu. “I believe this is the first step toward the comprehensive design of next-generation optoelectronic polymers that can provide high light-extraction efficiency without harming the environment.”
***
Reference
DOI: https://doi.org/10.1002/adfm.202404433
Authors: Seigo Watanabe1, Luca M. Cavinato2, Vladimir Calviv3, Richard van Rijn3, Rubén D. Costa2, and Kenichi Oyaizu1
Affiliations:
1Department of Applied Chemistry and Research Institute for Science and Engineering, Waseda University
2Chair of Biogenic Functional Materials, Technical University of Munich, TUM Campus Straubing for Biotechnology and Sustainability
3Applied Nanolayers B.V.
About Waseda University
Located in the heart of Tokyo, Waseda University is a leading private research university that has long been dedicated to academic excellence, innovative research, and civic engagement at both the local and global levels since 1882. The University has produced many changemakers in its history, including nine prime ministers and many leaders in business, science and technology, literature, sports, and film. Waseda has strong collaborations with overseas research institutions and is committed to advancing cutting-edge research and developing leaders who can contribute to the resolution of complex, global social issues. The University has set a target of achieving a zero-carbon campus by 2032, in line with the Sustainable Development Goals (SDGs) adopted by the United Nations in 2015.
To learn more about Waseda University, visit https://www.waseda.jp/top/en
About Professor Kenichi Oyaizu
Dr. Kenichi Oyaizu obtained a PhD degree from Waseda University in 1995. He has served as an Associate Professor since 2007. His research focuses on the development of polymers for electron-transfer-based organic electronics, for wet-type energy storage, transport, and conversion devices. He has published approximately 300 papers on these topics. Notably, he received the Award for Encouragement of Research in Polymer Science from The Society of Polymer Science, Japan, in 2001 and the Chemical Society of Japan Award for Young Chemists in 2002.
END