Since its discovery, in 1836, acetylene has emerged as an essential chemical compound in industry, widely used as a chemical building block and fuel. It has applications in the raw material for resins, such as vinyl chloride, welding gas, and illumination. Recent developments aimed at reducing the dependence on petroleum feedstocks have shown that acetylene is a promising platform molecule for producing various base chemicals. Additionally, polyacetylene, a crucial semiconducting material, is made from acetylene. Currently, acetylene is mainly produced through two methods: the calcium carbide process and cracking. The calcium carbide process involves reacting coal-derived coke and quicklime to form calcium carbide (CaC2), which is then reacted with water to produce acetylene. In cracking, the thermal decomposition of fossil fuels is used to make acetylene. Both methods depend heavily on fossil fuels, contributing significantly to the carbon footprint. Therefore, developing a new sustainable method that does not rely on fossil fuels is essential.
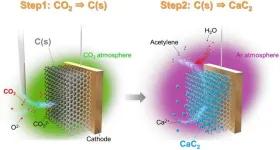
In a recent study, a team of Japanese researchers proposed an innovative electrochemical process using high-temperature molten salts. This method produces acetylene indirectly by the formation of CaC2 from carbon dioxide (CO2). In this process, CaC2 is obtained through electrodeposition and is subsequently reacted with water to yield acetylene. However, the current efficiency of this process is less than 10% in most conditions.
Addressing this issue, in a new study, the team, led by Assistant Professor Yuta Suzuki (first author) from Harris Science Research Institute, Doshisha University, and Professor Takuya Goto (corresponding author) from the Department of Science of Environment and Mathematical Modeling, Graduate School of Science and Engineering, Doshisha University, and including Dr. Tomohiro Isogai from the Technology and Innovation Center at Daikin Industries Ltd., developed a more efficient process with selective CaC2 formation. Dr. Suzuki explains, “To increase the current efficiency of acetylene formation, it is necessary to selectively produce CaC2 from CO2, which depends on controlling interfacial phenomena between the electrode and the molten salt electrolyte. One approach is to separate the reactions of carbon electrodeposition from CO2 and the Ca(II) ions reduction on the CO2-derived solid carbon”. Their study, an academia–industry collaboration, was made available online on June 11, 2024, and published in Volume 494 of the Chemical Engineering Journal on August 15, 2024.
In this method, acetylene is produced through a two-step electrolysis process. First, an electrolytic cell containing iron as the working electrode is immersed in a molten salt mixture (NaCl–KCl–CaCl2) at 823 K under a CO2 atmosphere. This results in the electrodeposition of carbon, from the CO2 onto the iron electrode, forming a carbon electrode. In the second step, this electrodeposited carbon is selectively converted to CaC2 under an inert Argon atmosphere, which can then be used to synthesize acetylene. This second electrolysis can be carried out either using the carbon electrode formed in the first step as is or the carbon residue after hydrolysis. In this study, the researchers utilized the carbon electrode from the first step.
This method achieves acetylene synthesis with a high current efficiency of 92%. Moreover, unlike conventional methods using fossil fuels, the CaC2 produced in this process does not contain sulfur and phosphorous in the product gas and contributes to carbon recycling by realizing highly efficient CaC2 production.
The researchers also investigated the mechanism of the formation of CaC2 and found that it was induced by the electrochemical reaction of Ca(II) ions at the carbon’s basal plane, rather than through interlayer reactions, representing a unique interfacial phenomenon. Highlighting the potential of this method, Prof. Goto remarks, “This method can lead to the realization of an acetylene-based chemical industry with acetylene made from CO2. This process will help build a new industry based on resource recycling without using fossil fuels.”
This study marks a significant step toward the sustainable synthesis of acetylene and related products.
About Assistant Professor Yuta Suzuki from Doshisha University, Japan
Yuta Suzuki is currently an Assistant Professor at the Harris Science Research Institute at Doshisha University. He obtained his M.S. and Ph.D. degrees from Doshisha University in 2018 and 2021, respectively. In 2023, he received the Outstanding Lecture Award of the New Reactor Subcommittee from the Atomic Energy Society of Japan. His research interests include Earth resource engineering, energy science, nanotechnology, sustainable chemistry, environmental chemistry, metals and resources production, and inorganic and coordination chemistry.
About Professor Takuya Goto from Doshisha University, Japan
Takuya Goto is currently a Professor at the Department of Science of Environment and Mathematical Modeling, Graduate School of Science and Engineering, Doshisha University. He obtained his Ph.D. in Energy Science from Kyoto University, Japan. He has about 100 publications with over 1000 citations. His research interests include nuclear engineering, inorganic chemistry, and electrochemistry.
Funding information
JSPS KAKENHI Grant Number JP22K14700, Carbon Recycling Fund Institute, and Steel Foundation for Environmental Protection Technology partially supported this work.
Media contact:
Organization for Research Initiatives & Development
Doshisha University
Kyotanabe, Kyoto 610-0394, JAPAN
E-mail:jt-ura@mail.doshisha.ac.jp
END