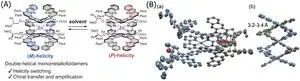
The deoxyribonucleic acid or DNA, the molecular system that carries the genetic information of living organisms, can transcribe and amplify information using its two helical strands. Creating such artificial molecular systems that match or surpass DNA in functionality is of great interest to scientists. Double-helical foldamers are one such molecular system.
Helical foldamers are a class of artificial molecules that fold into well-defined helical structures like helices found in proteins and nucleic acids. They have garnered considerable attention as stimuli-responsive switchable molecules, tuneable chiral materials, and cooperative supramolecular systems due to their chiral and conformational switching properties. Double-helical foldamers exhibit not only even stronger chiral properties but also unique properties, such as the transcription of chiral information from one chiral strand to another without chiral properties, enabling potential applications in higher-order structural control related to replication, like nucleic acids. However, the artificial control of the chiral switching properties of such artificial molecules remains challenging due to the difficulty in balancing the dynamic properties required for switching and stability. Although various helical molecules have been developed in the past, reversal of twist direction in double-helix molecules and supramolecules has rarely been reported.
In a breakthrough, a team of researchers from Tokyo University of Science, Japan, led by Professor Hidetoshi Kawai from the Department of Chemistry, Faculty of Science, and including Mr. Kotaro Matsumura from the Department of Chemistry, developed a novel mechanical motif, called double-helical monometallofoldamers with controllable chiral switching. Prof. Kawai explains, “In this research, we succeeded in synthesizing a double helical mononuclear complex bridged with a single metal cation in the center of the helices to balance both stability and dynamic properties. These structures can undergo inversion switching by changing the left and right winding directions of both helix strands using different solvents.” Their study was published in the Journal of the American Chemical Society on July 19, 2024.
The researchers synthesized the double-helical monometallofoldamers from two bipyridine-type strands with L-shaped units, which after forming a complex with a zinc cation formed double-helical structures. X-ray crystallography revealed the double-helical structures with a metal cation in their center. The researchers investigated the switchability of monometallofoldamers in response to external stimuli and found that helix terminals of the double-helical form can unfold in solutions, resulting in the open form, favored at high temperatures, and refold to the double-helical form, favored at low temperatures.
Interestingly, the helicity of the double-helical monometallofoldamer with chiral chains can be controlled in response to achiral solvents. For example, in non-polar solvents (toluene, hexane, Et2O), it becomes left-handed or M-form, and in Lewis basic solvents (acetone, DMSO), it becomes right-handed or the P-form. The conformation of chiral chains introduced into the helix strands was found to be important for this M/P switching. Furthermore, they found that when a helix strand with chiral chains is mixed with a strand without chiral chains, the winding direction of the helix is transmitted and amplified to the achiral strand without chiral chains, with the helicity inversion ability maintained.
Emphasizing the significance of this new molecule, Mr. Matsumura says, “Our synthesized double-helical monometallofoldamers has the potential to be applied to new switching chiral materials that output diverse chiral properties by small inputs and can be used to develop chiral sensors. In addition, we expect that this novel molecular structure will lead to facilitate the genesis of deracemized and organized supramolecular systems as those found in nature by transmitting and amplifying their superior chiral properties.”
Overall, this study marks a significant step towards realizing artificial controllable double-helical structures, paving the way for novel high-order molecular systems and molecular information processing.
***
Reference
DOI: 10.1021/jacs.4c06560
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Professor Hidetoshi Kawai from Tokyo University of Science
Hidetoshi Kawai is currently a Professor at the Department of Chemistry, Faculty of Science at Tokyo University of Science. He obtained his Ph.D. from Hokkaido University in 2000. He has over 130 publications with over 2500 citations. At Tokyo University of Science, he also leads the Kawai lab. Additionally, he served as a guest lecturer at the Université de Strasbourg, France in 2016. His research interests include supramolecular chemistry, structural organic chemistry, high-efficiency molecular conversion, and self-amplifying molecules among others.
Funding information
This work was supported by the JSPS KAKENHI (Grant 461 Numbers JP20K05478 and JP24K08385 for H.K. and 462 JP16J08668 for K. T.) and JST SPRING (Grant Number 463 JPMJSP2151 for K. M.).
END