A new study details how a nasal spray formulated by investigators from Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, may work to protect against viral and bacterial respiratory infections. Based on their preclinical studies, the researchers say the broad-spectrum nasal spray is long-lasting, safe, and, if validated in humans, could play a key role in reducing respiratory diseases and safeguarding public health against new threats. Their results are published in the journal Advanced Materials.
“The COVID pandemic showed us what respiratory pathogens can do to humanity in a very short time. That threat hasn’t gone away,” said co-senior author Jeffrey Karp, PhD, distinguished chair in Anesthesiology at Brigham and Women’s Hospital. “Not only do we have the flu to deal with seasonally, but we now have COVID, too.”
Influenza and COVID-19 infections cause thousands of deaths and hundreds of thousands of cases of severe disease every year. Milder infections cause significant discomfort, resulting in missed work or school.
Vaccines against these viruses can be beneficial, but they’re imperfect. Vaccinated people still get infected and spread the infection to others. Masks are also helpful but aren’t perfect, either — they can leak, and many people wear them improperly or choose not to wear them at all.
“We need new, additional ways to protect ourselves and reduce the transmission of the disease,” Karp said.
Most viruses enter our system through the nose. When we catch an airborne infection like the flu and COVID, we breathe out tiny droplets of fluids that contain the pathogen. Healthy people around us breathe in these pathogen-containing droplets, which attach inside their nose and infect the cells that line the nasal passageways. The pathogen replicates and can be released back into the air when an individual who is sick, whether they know it or not, sneezes, coughs, laughs, sings, or even just breathes.
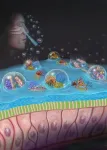
The new study details the research team’s efforts to create a nasal spray to defend against airborne respiratory illness. “The spray, called Pathogen Capture and Neutralizing Spray (PCANS) in the paper, was developed using ingredients from the FDA’s Inactive Ingredient Database (IID), which have been previously used in approved nasal sprays, or from the Generally Recognized as Safe (GRAS) list of the FDA,” said co-senior author Nitin Joshi, PhD, an Assistant Professor of Anesthesiology at Brigham and Women’s Hospital. “We developed a drug-free formulation using these compounds to block germs in three ways — PCANS forms a gel-like matrix that traps respiratory droplets, immobilizes the germs, and effectively neutralizes them, preventing infection.”
The researchers did the experiments detailed in the study under laboratory settings. They have not studied PCANS directly in humans. The researchers developed the formulation and studied its ability to capture respiratory droplets in a 3D-printed replica of a human nose. They showed that when sprayed in the nasal cavity replica, PCANS captured twice as many droplets as mucus alone.
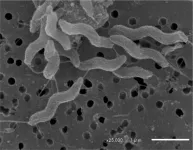
“PCANS forms a gel, increasing its mechanical strength by a hundred times, forming a solid barrier,” said primary author John Joseph, PhD, a former postdoctoral fellow at Brigham and Women’s Hospital. “It blocked and neutralized almost 100% of all viruses and bacteria we tested, including Influenza, SARS-CoV-2, RSV, adenovirus, K Pneumonia and more.”
Experiments in mice showed that a single dose of the PCANS nasal spray could effectively block infection from an influenza virus (PR8) at 25 times the lethal dose. Virus levels in the lungs were reduced by >99.99%, and the inflammatory cells and cytokines in the lungs of PCANS-treated animals were normal.
“The formulation’s ability to inactivate a broad spectrum of pathogens, including the deadly PR8 influenza virus, demonstrates its high effectiveness,” said co-senior author Yohannes Tesfaigzi, PhD, AstraZeneca Professor of Medicine in the Field of Respiratory and Inflammatory Diseases at Brigham and Women’s Hospital. “In a rigorous mouse model study, prophylactic treatment with PCANS demonstrated exceptional efficacy, with treated mice exhibiting complete protection, while the untreated group showed no such benefit.”
While the study’s limitations include the lack of human studies of PCANS, it provides a strong foundation for future research to explore the full potential of PCANS in a broader context. The researchers are exploring whether PCANS can also block allergens, opening a potential new avenue for allergy relief.
Authorship: BWH authors include John Joseph, Hela Mary Baby, Joselyn Rojas Quintero, Yohannes A. Mebratu, Purna Shah, Kabir Swain, Dongtak Lee, Shahdeep Kaur, Xiang-Ling Li, John Mwangi, Olivia Snapper, Eli Agus, Sruthi Ranganathan, Julian Kage, Jingjing Gao, Anthony Yu, James N. Luo, Yohannes Tesfaigzi, Jeffrey M. Karp and Nitin Joshi. Authors also include Remya Nair (Harvard Medical School), Devin Kenney, Florian Douam, Eshant Bhatia and Dongsung Park. Joseph, Baby, and Quintero contributed equally to this work.
Disclosures: John Joseph, Helna Mary Baby, Yohannes Tesfaigzi, Jeffrey Karp and Nitin Joshi have one pending patent based on the PCANS formulation described in this manuscript. BWH has licensed the technology to Akita Biosciences which has now commercialized PCANS as Profi Nasal Spray, a personal care product, and it is readily available to the public. Joshi and Karp are paid consultants, scientific advisory board members, and hold equity in Akita Biosciences, and the company, BWH, Joshi and Karp may benefit financially if the IP is further validated. Karp has been a paid consultant or equity holder for multiple other biotechnology companies.
Funding: This study was supported by funding from the Gillian Reny Stepping Strong Center for Trauma Innovation at Brigham and Women’s Hospital, and the Department of Anesthesiology, Perioperative, and Pain Medicine at Brigham and Women’s Hospital.
Paper cited: Joseph, J et al. “Toward a Radically Simple Multi-Modal Nasal Spray for Preventing Respiratory Infections” Journal DOI: 10.1002/adma.202406348
###
About Mass General Brigham
Mass General Brigham is an integrated academic health care system, uniting great minds to solve the hardest problems in medicine for our communities and the world. Mass General Brigham connects a full continuum of care across a system of academic medical centers, community and specialty hospitals, a health insurance plan, physician networks, community health centers, home care, and long-term care services. Mass General Brigham is a nonprofit organization committed to patient care, research, teaching, and service to the community. In addition, Mass General Brigham is one of the nation’s leading biomedical research organizations with several Harvard Medical School teaching hospitals. For more information, please visit massgeneralbrigham.org.
END