You may not be familiar with hydrogen sulfide, a colorless gas that smells like rotten eggs, and is produced naturally from decaying matter. However, this gas is lethal to breathe in, and hydrogen sulfide present in high concentrations can cause death very rapidly. Its relative density is also greater than air, causing it to accumulate at lower altitudes and posing an enormous threat to workers at sites, such as manholes, sewage systems and mining operations.
Why is hydrogen sulfide so dangerous? It binds strongly to the heme-containing cytochrome c oxidase (CcO) enzyme and blocks the cellular process of aerobic (oxygen-dependent) respiration. What is even more concerning is that as of now, there is no identified antidote that can treat hydrogen sulfide poisoning. Hence, there is an urgent need to develop therapeutic agents that can be stored for long durations and are effective against hydrogen sulfide poisoning immediately.
Now, a study led by Professor Hiroaki Kitagishi at Doshisha University and published online on December 10, 2024, in Scientific Reports has proposed a novel antidote for hydrogen sulfide poisoning. Atsuki Nakagami, Ph.D. students in the Department of Applied Chemistry at the Graduate School of Doshisha University, Dr. Qiyue Mao, Specially Appointed Assistant Professor at Doshisha University, Associate Professor Masaki Horitani at the Faculty of Agriculture, Saga University, and Professor Masahito Kodera at the Faculty of Science and Engineering, Doshisha University, also contributed to the results of this study. They decided to tackle this problem by using artificial heme-model compounds that would have a higher affinity towards hydrogen sulfide than the native hemes present in our bodies.
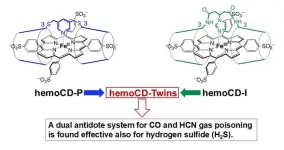
Providing more context to their approach, Prof. Kitagishi explains, “We have developed and studied synthetic heme-model compounds (hemoCDs) over the last two decades. The series of hemoCDs, which consist of porphyrin and cyclodextrins, is our original heme-model system that realizes the biological functions of hemes (like hemoglobin) while using completely synthetic materials.” Previously, Prof. Kitagishi and his collaborators had used two novel hemoCDs dubbed “hemoCD-Twins”—met-hemoCD-P and met-hemoCD-I—to successfully treat carbon monoxide and hydrogen cyanide poisoning in mice.
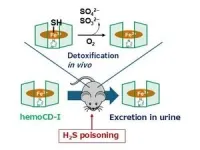
Now, they decided to test if these two complexes had the potential to ‘scavenge’ hydrogen sulfide in an aqueous medium. Interestingly, they found that met-hemoCD-I in particular had a very high affinity for hydrogen sulfide under normal physiological conditions—almost 10 times higher than that of human met-hemoglobin. Met-hemoCD-I was able to convert toxic hydrogen sulfide into nontoxic sulfite and sulfate ions, indicating that it could be used to treat hydrogen sulfide poisoning.
To test this antidote, they injected hydrogen sulfide-treated mice with met-hemoCD-I. The results were very promising—mice injected with met-hemoCD-I showed improved survival rates compared to mice that were not given the antidote. Additionally, CcO activity in the brain and heart tissues (which had decreased because of poisoning) recovered and returned to normal.
Another aspect of met-hemoCD-I that makes it a very promising antidote is its demonstrated safety—it was found that injected met-hemoCD-I was excreted in the urine of the rats without undergoing any chemical decomposition in their body.
The results of this study show that hemoCD-Twins could be used as a powerful antidote to treat carbon monoxide, hydrogen cyanide, and now hydrogen sulfide poisoning without the risk of any side effects. Explaining their vision for this treatment, Prof. Kitagishi says, “Using hemoCD-Twins, we can provide one powerful solution for multiple gas poisoning, even if the cause of poisoning is unknown. Worldwide, we still do not have an actual solution for accidentally occurring gas poisoning—we would like to supply hemoCD to fulfil this unmet medical need.”
In the future, they hope to bring this rapid and effective treatment to clinics and other medical settings. “We will proceed with non-clinical and clinical trials in cooperation with medical doctors in order to implement this compound as a therapeutic agent actually used in the world,” adds Prof. Kitagishi.
We are confident that this antidote will prove invaluable for improving the safety of workers and rescue personnel around the world!
About Professor Hiroaki Kitagishi from Doshisha University, Japan
Hiroaki Kitagishi is a Professor in the Department of Molecular Chemistry and Biochemistry at Doshisha University, Kyoto, Japan. He received his PhD from Doshisha University and was a postdoctoral fellow at Osaka University, before joining Doshisha University as an Assistant Professor in 2008. His research interests include supramolecular chemistry, bioinorganic chemistry, carbon monoxide, heme, cyclodextrins, and porphyrin. He has published over 100 papers in the course of his career and accumulated around 2,000 citations for the same.
Funding information
This work was funded by JSPS KAKENHI (22H02097 and 24K01640), Kyoto University in the Translational Research Program from AMED (23ym0126814 and 24ym0126808), and JST (JPMJSF2305, JPMJSP2129). Part of this work was conducted at the Institute for Molecular Science, supported by Nanotechnology Platform Program < Molecule and Material Synthesis > JPMXP1224MS1025 (to M.H.) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Media contact:
Organization for Research Initiatives & Development
Doshisha University
Kyotanabe, Kyoto 610-0394, JAPAN
E-mail:jt-ura@mail.doshisha.ac.jp
END