(Press-News.org) Viruses have led scientists at Washington University School of Medicine in St. Louis to the discovery of a security system in host cells.
Viruses that cause disease in animals beat the security system millennia ago. But now that researchers are aware of it, they can explore the possibility of bringing the system back into play in the fight against diseases such as sudden acute respiratory syndrome (SARS), West Nile virus, dengue and yellow fever.
The findings, published in Nature, solve a 35-year-old mystery that began when National Institutes of Health researcher Bernard Moss, MD, PhD, noticed that poxviruses put chemical "caps" on particular spots in every piece of genetic material transcribed from their DNA. That transcribed material is RNA; to reproduce, viruses need to trick the host cell into making viral proteins from this RNA.
Noting evidence that the host cell puts caps on its own RNA in identical positions, Moss theorized that the caps might be a way for cells to distinguish between their RNA and that of an invader. He guessed the caps might serve as a sort of fake identification badge for the virus' RNA, allowing it to bypass host cell security systems primed to attack any RNA lacking the caps.
Since Moss's study, scientists have learned that some viruses have strategies for stealing RNA caps from host cells and putting them on their own RNA. Several disease-causing viruses have to make their own caps, including:
poxviruses, which cause smallpox
flaviviruses, which cause West Nile encephalitis, yellow fever and dengue;
rhabdoviruses, which cause rabies;
coronaviruses, which cause SARS;
reoviruses, which cause mild respiratory distress or diarrhea.
Scientists also learned that one of the chemical caps added to RNA helps stabilize it, preventing the RNA from breaking down. However, despite years of research, the purpose of another cap, added near the beginning of every RNA strand in a position scientists refer to as 2' (two prime), was a persistent mystery.
The new paper from the laboratory of senior author Michael S. Diamond, MD, PhD, solves that puzzle and confirms Moss' speculation. The study used a mutant form of the West Nile virus created by Pei-Yong Shi, PhD, now a researcher at the Novartis Institute for Tropical Diseases. The mutant strain can attach the cap that keeps RNA stable but is unable to add the 2' cap. When Diamond, professor of medicine, pathology and immunology, and molecular microbiology at Washington University School of Medicine, infected mice with this mutant virus, it could not cause disease.
Next, scientists injected the mutant virus into mice lacking the receptors for interferons. These proteins are important players in defensive reactions to invading viruses within the cell, a branch of the immune system known as intrinsic immunity. The mutant virus made these mice sick, suggesting that intrinsic immunity stops the mutant viruses in normal mice, and that the 2' cap was helping normal viruses evade this part of the immune system.
Researchers recently identified a gene, IFIT2, that is activated by interferons, has mild antiviral effects against West Nile virus and seems to have potential connections to translation of RNA into proteins. When Diamond turned IFIT2 levels up in cell culture and exposed it to the mutant West Nile virus, the mutant virus could barely replicate. Tests of a mutant poxvirus and a mutant coronavirus that could not attach the 2' cap produced similar results. Knocking out a related gene in mice, IFIT1, allowed the mutant virus to evade intrinsic immunity and cause infection when it was injected into the brain.
"Now that we know what this cap is used for, we can look at the question of whether the human and viral enzymes that put the cap on are sufficiently different," says Diamond. "If they are, we may be able to design inhibitors that prevent viruses from capping their RNA and make it much harder for them to replicate once the intrinsic immune system is activated."
###
Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin T-Y, Schneller S, Zust R, Dong H, Thiel V, Sen GC, Fensterl V, Klimstra WB, Pierson TC, Buller RM, Gale JR M, Shi P-Y, Diamond, MS. 2'0 methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature, Nov. 18, 2010.
Funding from the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research, the Pacific Northwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research, and the Northeast Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research supported this research.
Washington University School of Medicine's 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children's hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked fourth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children's hospitals, the School of Medicine is linked to BJC HealthCare.
END
CHAPEL HILL, N.C. -- Like all organisms, bacteria must compete for resources to survive, even if it means a fight to the death.
New research led by scientists from the University of North Carolina at Chapel Hill School of Medicine and the University of California, Santa Barbara, describes new complexities in the close chemical combat waged among bacteria.
And the findings from this microscopic war zone may have implications for human health and survival.
"It has been known for a long time that bacteria can produce toxins that they release into their surroundings ...
NEW YORK, November, 17, 2010 — An international, multi-center study has revealed the discovery of a novel oncogene that is associated with uveal melanoma, the most common form of eye cancer. Researchers have isolated an oncogene called GNA11 and have found that it is present in more than 40 percent of tumor samples taken from patients with uveal melanoma. The findings are being published early online November 17, 2010 in the New England Journal of Medicine and will appear in the December 2, 2010, print issue.
"These findings are significant because we now have a much ...
Left ventricular non-compaction (LVNC), a cardiomyopathy about which little is fully understood, is associated with heart failure (HF), stroke and ventricular arrhythmias, according to a study to be presented Nov. 17 at the 2010 American Heart Association (AHA) Scientific Sessions in Chicago. The researchers also will report that advanced imaging technologies reveal that developing these cardiac risks appear to progress over time in patients with LVNC.
LVNC is an inherited heart muscle condition in which the muscular wall of the left ventricle appears to be spongy and ...
Stop using racial profiling, says Professor William Press from the University of Texas at Austin. He claims that as well as being politically and ethically questionable, racial profiling does no better in helping law enforcement officials in their task of catching terrorists than standard uniform random sampling techniques. This is the topic of a paper publishing today in Significance, the magazine of the Royal Statistical Society and the American Statistical Association.
Racial profiling rests on the idea that people from particular racial or ethnic groups are more ...
Northwestern Medicine physician researchers are revolutionizing treatment of cardiovascular disease by utilizing patients' own stem cells to regenerate heart and vascular tissue. Northwestern Medicine is the lead site for a study examining stem cell transplantation as treatment for critical limb ischemia. Chief investigator Douglas Losordo, MD, director of the Program in Cardiovascular Regenerative Medicine at Northwestern Memorial Hospital and the Eileen M. Foell Professor of Heart Research of Northwestern University's Feinberg School of Medicine, will present the findings ...
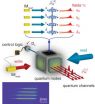
PASADENA, Calif. — Researchers at the California Institute of Technology (Caltech) have demonstrated quantum entanglement for a quantum state stored in four spatially distinct atomic memories.
Their work, described in the November 18 issue of the journal Nature, also demonstrated a quantum interface between the atomic memories—which represent something akin to a computer "hard drive" for entanglement—and four beams of light, thereby enabling the four-fold entanglement to be distributed by photons across quantum networks. The research represents an important achievement ...
A new anti-cancer drug aimed at vitamin D receptors on cancer cells has prompted encouraging responses in the levels of PSA (prostate specific antigen) in men with prostate cancer that has become resistant to hormonal therapies.
Results of the phase II(a) clinical trial will be presented at the 22nd EORTC-NCI-AACR [1] Symposium on Molecular Targets and Cancer Therapeutics in Berlin today (Thursday). The trial found that when the new drug, inecalcitol, was combined with the existing, current therapy (docetaxel and prednisone) 83% of patients responded to the treatment ...
Scientists in Germany have developed a way of smuggling an anti-cancer drug past the protective blood-brain barrier and into brain tumours and metastases using a nanocarrier – a tiny capsule specially designed to pass through cell membranes and deliver its anti-cancer drug to the cancer cell.
The blood-brain barrier is formed by a network of closely sealed endothelial cells in the brain's capillaries, and it expresses a high level of proteins that pump foreign molecules away from the brain, while allowing others (such as glucose and insulin) that are necessary to the ...
In the movie Angels and Demons, scientists have solved one of the most perplexing scientific problems: the capture and storage of antimatter. In real life, trapping atomic antimatter has never been accomplished, until now.
A team made up of researchers from the University of Calgary, institutions across Canada and around the world have discovered how to trap atomic antimatter and the results of their discovery is published in the journal Nature.
"This is a major discovery. It could enable experiments that result in dramatic changes to the current view of fundamental ...
A new drug that targets proteins responsible for helping cancer cells to repair damage to their DNA has shown promising anti-tumour activity in its first trial in humans. Some patients with a range of solid tumours, many of whom had been treated unsuccessfully for their cancer with other therapies, have seen their tumours shrink or stabilise for periods of between 46 days to more than a year. The research will be presented at the 22nd EORTC-NCI-AACR [1] Symposium on Molecular Targets and Cancer Therapeutics in Berlin today (Thursday).
Laboratory studies of the drug, MK-4827, ...