It is hoped that the number of children suffering severe aftereffects resulting from congenital CMV infection will decrease in the future.
The research group included the following members:
Doctor YAMADA Hideto (Director of the Center for Recurrent Pregnancy Loss and Genome Medical Center, Teine Keijinkai Hospital, visiting professor at Osaka University and former professor of the Department of Obstetrics and Gynecology, Kobe University Graduate School of Medicine) Associate Professor TANIMURA Kenji (Department of Obstetrics and Gynecology, Kobe University Graduate School of Medicine), Associate Professor FUJIOKA Kazumichi (Department of Pediatrics, Kobe University Graduate School of Medicine) Professor MORIOKA Ichiro (Department of Pediatrics and Child Health, Nihon University School of Medicine) The pre-proof paper on research results was published online in the Journal of Reproductive Immunology on December 16 2020, with the final version being made available on January 8 2021.
Main Points
When CMV infects fetuses, it can cause severe handicaps such as hearing difficulties, as well as mental and physical development disorders. In particular, 90% of babies who are born with the symptoms of congenital CMV infection experience serious long-term aftereffects. It is known that hearing difficulties and delays in mental development can be ameliorated in babies born with symptoms of CMV infection, if they are given prompt treatment with antiviral drugs after birth. Although there have been some attempts to treat fetuses diagnosed with congenital CMV infection during the gestational period (fetal therapies), there is currently no established method of treatment for babies before they are born. Until now, there has been no research carried out into the effectiveness of combining fetal treatment with neonatal treatment. This research team has shown for the first time in the world, that a combination of fetal and neonatal treatments is more likely to lessen the aftereffects of congenital CMV infection in infants than neonatal treatment alone. Research Background
Cytomegalovirus (CMV) can infect babies while they are in the uterus. Congenital CMV infection can cause severe aftereffects in these children such as hearing difficulties, and mental and physical developmental disorders. This is a big issue worldwide, for example, it is estimated that around 1000 babies are born with congenital CMV infection each year in Japan. Fetal growth restriction (*1), microcephaly (*2), ventriculomegaly (*3), hepatomegaly (*4), pleural effusion and peritoneal effusion (*5) are among the characteristic symptoms of congenital CMV infection. Approximately 90% of infants who experience these clinical manifestations are left with the aforementioned severe aftereffects.
In recent years, it has been discovered that treating newborns with these clinical manifestations of congenital CMV infection with the antiviral drug, Valganciclovir, can improve not only hearing issues but also reduce delays in mental and physical development. In Japan, a clinical trial is ongoing to approve the neonatal therapies as treatments covered by public health insurance.
On the other hand, there are some cases where clear clinical manifestations of CMV infection can be diagnosed in fetuses via ultrasound while they are still in the uterus. It is supposed that infants who exhibit these symptoms in the uterus are more likely to experience more severe aftereffects than infants who are diagnosed with congenital CMV infection after being born.
Up until now, there have been some reports on the effectiveness of administering immunoglobulin blood products (*6) during the gestational period via the mother's bloodstream, the amniotic fluid, the umbilical cord or into the fetus's abdomen, to treat fetuses diagnosed with symptomatic congenital CMV infection. A previous study has also looked at administering valacyclovir, which is an antiviral drug used against herpesvirus, to pregnant women carrying fetuses with CMV.
However, these studies included fetuses with no clear clinical manifestations of CMV infection (asymptomatic cases) or those with only mild symptoms. Therefore no clear answer has been obtained as to whether these treatments are effective for fetuses with serious forms of congenital CMV infection. Unfortunately, as a consequence of this, there is currently no established fetal treatment method.
In 1996, Doctor Yamada et al. became the first in the world to try treating symptomatic congenital CMV infection with immunoglobulin fetal therapy (J Perinatol 1998). After that, the results of a multicenter study showing the effectiveness of immunoglobulin fetal treatment in 12 cases were published (J Perinatol 2012). However, up until now research has focused on fetal treatment and neonatal treatment separately. There has been no research published on the effectiveness of a combined fetal and neonatal treatment approach.
Therefore this research group sought to investigate for the first time in the world, whether or not an integrated fetal and neonatal treatment approach (immunoglobulin therapy while in the uterus and antiviral drug therapy after birth) could lessen the severity of aftereffects experienced by infants with congenital CMV infection.
Research Findings
The researchers carried out fetal therapy (FT) and neonatal therapy (NT) at Kobe University Hospital. The pregnant women and their husbands were given a thorough explanation of the treatments and their consent was obtained prior to the study's commencement.
The presence of congenital CMV infection during the gestational period was determined either through ultrasound examinations in which characteristic symptoms of congenital CMV infection were observed, or through CMV-DNA PCR analysis of amniotic fluid sampled from the pregnant women. In CMV positive cases, the pregnant women and their partners were asked whether or not they wished to receive FT. This FT was administered to those who agreed; immunoglobulin was administered either in the form of an injection into the abdomen of the fetus or via intravenous injection to the mothers. If the FT was effective, the treatment was continued. If it was found to be ineffective, the researchers considered inducing birth once the fetus was over 32 weeks with a weight of over 1200g, in order to start neonatal therapy as soon as possible.
All babies who received FT were given comprehensive examinations after being born, including ultrasound examinations, CT scans, Auditory Brainstem Response tests (*7), and ophthalmoscopes (eye tests). From these results, babies were given NT with the antiviral drug Valganciclovir (which has been shown to be effective against CMV), if they exhibited clinical manifestations of congenital CMV infection and PCR analysis of their urine was positive for CMV. Babies who did not show any symptoms of congenital CMV infection were not given NT, even if the PCR test result was positive.
On the other hand, some cases could only receive NT. This included cases where babies had been delivered at another hospital, been diagnosed with congenital CMV infection and then been transferred to the Department of Pediatrics at Kobe University, as well as cases where the parents refused FT for their diagnosed baby, and where the baby was born too early to receive FT. In addition, to make sure no cases of CMV infection were overlooked, PCR tests on the urine of all newborns delivered at Kobe University Hospital and several related hospitals were also carried out. Those newborns who were found to be CMV positive after the PCR test and also demonstrated clinical manifestations of the infection after thorough testing only received NT.
The research team conducted a long-term follow-up on the two groups of children: Group 1 (who received both FT and NT or who only received FT) and Group 2 (who only received NT). Between the ages 1.5 and 3 years old, periodical assessments were carried out to investigate whether or not each child had hearing loss, and if so, whether it was in one ear or both, as well as examinations to determine if there were any mental abnormalities. Based on these examinations and a Developmental Quotient (*8), neurological prognosis was divided into 3 categories: normal, mild impairments, and severe impairments. The researchers investigated whether there was a difference in these prognoses between Group 1 and Group 2 infants.
In the ten year period between 2009 and 2019, 15 cases received FT. In 4 of the 6 cases with fetal growth restriction, fetal growth increased after receiving FT. In 1 case both ventriculomegaly and hepatomegaly disappeared after FT, and in another case peritoneal effusion temporarily disappeared. Furthermore, the amount of CMV in the amniotic fluid either decreased or disappeared in 7 of the cases, and in 1 case CMV in the peritoneal fluid disappeared. Sadly, in 2 cases the babies died soon after birth due to respiratory failure caused by fluid build-up and insufficiently developed lungs. On the other hand, 1 of the cases did not require neonatal treatment, because, even though the post-birth PCR test was positive for CMV, the ventriculomegaly and hepatomegaly that they experienced during the gestational period had disappeared and they had no other symptoms of CMV infection. The remaining 12 cases received both FT and NT. Of the 19 cases receiving only NT in this ten year period, one was born prematurely at 24 weeks and sadly died one month later, so NT could not be completed.
Regarding the comparison of infants' neurological prognoses, some cases were omitted from the final analysis of the longitudinal data. These included cases where the periodical assessments at 1.5 years old could not be carried out for reasons such as the infant's death, not reaching the age for the assessments and the parents' refusal (4 cases in the FT group, and 3 in the NT only group). In the NT only group, two cases had chorioretinitis (*9) as the only manifestation of CMV infection, and as this could not be diagnosed during the gestational period, the infants did not receive fetal treatment. Therefore these two cases were also omitted.
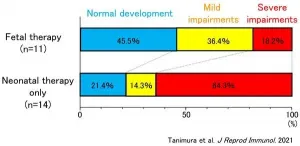
The percentage of infants who demonstrated normal development at ages 1.5 and 3 was 45.5% for the FT group (5 out of 11) and 21.4% for the NT only group (3 out of 14). Although the percentage for the FT group was higher, no statistically significant difference was found. However, in terms of the percentage of children with severe impairments, there was a clear statistical difference. In the NT only group 64.3% (9 out 14) had severe impairments, however this percentage was significantly lower for the FT group at only 18.2% (2 out of 11) (see Figure).
Further Research
It is supposed that infants who are diagnosed with congenital CMV infection during the gestational period have more severe symptoms than those who are diagnosed with it after being born. In a world first, the results of this research have shown the possibility that the long term neurological outcomes of fetuses diagnosed with congenital CMV infection can be improved via a combination of immunoglobulin fetal therapy and neonatal therapy with antiviral drugs.
The next issue is that a double-blind trial (*10) needs to be carried out in order to prove the effectiveness of treatment methods using drugs. However, it would be extremely unethical to use a placebo in cases where congenital CMV infection is detected during the gestational period and it is clear that without treatment the prognosis will worsen. After a new law related to clinical trials was passed, the fetal therapy method in this study became available but only as a treatment not covered by public health insurance. Therefore, this presents an issue from a financial point of view.
It is hoped that the results of this study will lead to further research into the use of immunoglobulin and other drugs in fetal therapy for congenital CMV infection, and that this will enable such treatments to be offered under public health insurance. This would hopefully lead to a decrease in the number of children affected by the aftereffects of this disease.
INFORMATION:
Glossary
1. Fetal growth restriction: A condition where the fetus's estimated weight is lower than the standard weight for the gestational weeks. It can be caused by various factors such as a viral infection, chromosomal abnormalities or dysfunction of the placenta.
2. Microcephaly: This is where the baby's head is smaller than usual due to CMV damaging the brain.
3. Ventriculomegaly: Damage to the brain resulting from CMV causes the brain structure to become smaller. This causes the cerebral ventricles, which are spaces for cerebrospinal fluid in the brain, to become larger than normal.
4. Hepatomegaly: CMV causes inflammation in the liver, causing the organ to become larger than normal.
5. Pleural and peritoneal effusion: This is a condition where fluid builds up in abdomen and chest due to the damage which CMV does to the liver, which also causes hypoproteinemia (very low levels of protein in the blood).
6. Immunoglobulin products: These are blood products that contain large amounts of the antibodies that our bodies use to protect us from pathogens. The fetal therapy carried out as part of this study used immunoglobulin products containing a large amount of antibodies that are especially effective against CMV.
7. Auditory Brainstem Response (ABR) Test: A hearing test carried out on patients who are unable to or have difficulty responding to sounds in a regular hearing test (e.g. newborn babies). The patient taking the test is given a series of sounds while electrodes measure how the brainstem responds to these sounds and records the results in a waveform.
8. Developmental Quotient: Criteria for quantifying children's development. There are many such evaluation methods; this study used the Kyoto Scale of Psychological Development.
9. Chorioretinitis: An eye condition where the retina, which is located behind the eyeball, and a membrane called the choroid are inflamed. This results in vision impairment.
10. Double-blind trial: A trial in which one group is administered a drug and the other group receives a placebo. It is called a double-blind trial because neither the doctors nor the patients know who is receiving the actual drug and who is receiving the placebo. Afterwards, the effectiveness of the drug as a treatment method is evaluated by comparing the two groups to see which percentage had improved symptoms.
Journal Information
Title
"Immunoglobulin fetal therapy and neonatal therapy with antiviral drugs improve neurological outcome of infants with symptomatic congenital cytomegalovirus infection"
DOI:10.1016/j.jri.2020.103263
Authors
Kenji Tanimura, Yutoku Shi, Akiko Uchida, Mizuki Uenaka, Hitomi Imafuku, Toshihiko Ikuta, Kazumichi Fujioka, Ichiro Morioka, Masashi Deguchi, Toshio Minematsu, and Hideto Yamada
Journal
Journal of Reproductive Immunology