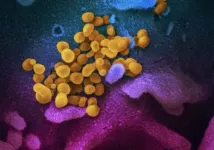
As the COVID-19 pandemic enters its second year, scientists are still working to understand how the new strain of coronavirus evolved, and how it became so much more dangerous than other coronaviruses, which humans have been living alongside for millennia.
Virologists and epidemiologists worldwide have speculated for months that a protein called ORF8 likely holds the answer, and a recent study by Berkeley Lab scientists has helped confirm this hypothesis.
In a paper published in mBio, lead author Russell Neches and his colleagues show that ORF8 evolved from another coronavirus protein called ORF7a, and that both proteins have folds similar to that of a human antibody. This finding helps to explain how the virus avoids immune detection and is able to escalate into a severe infection in some hosts.
"By exploring the structural and functional characteristics of ORF8, and using supercomputers to look at the genomes of over 200,000 viruses, we discovered a striking and highly unusual evolutionary strategy," said co-author Nikos Kyrpides, a computational biologist at the DOE Joint Genome Institute (JGI). "Amazingly, it seems that within the SARS clade, the gene encoding ORF7a is used as a 'template' gene, remaining stable, with a duplicate copy of this gene evolving to a point almost beyond recognition." SARS-CoV-2 arose and exploded into a pandemic when a SARS strain's duplicate ORF7a gene happened to mutate leading to a new protein (which we now call ORF8) that gave it the ability to interfere with immune cells.
According to the team, a similar event occurred in the SARS-CoV strain that caused the SARS epidemic in the early 2000s. In that instance, a copy of the ORF7a gene split into two, resulting in ORF8a and ORF8b proteins.
Christos Ouzounis, senior author of the study and a JGI affiliate scientist, noted that the connection between ORF8 and ORF7a was initially quite difficult to make, due to how little was known about this set of genes and their encoded proteins compared with the existing knowledge about surface proteins (such as the infamous spike protein), and because ORF8 and ORF7a currently seem wildly different. ORF7a is highly stable, mutation resistant protein that interacts with very few mammalian host proteins, whereas ORF8 is encoded by the most mutation prone gene in the viral genome, and is now known to be involved in dozens of interactions in the human body.
"Our findings - and their confirmation by parallel sequence and structure studies - reveal ORF8 to be an evolutionary hotspot in the SARS lineage. The lack of knowledge about the role of these genes has diverted attention to the more well-understood genes, but we now know more about this gene and hopefully it will receive more attention from the community," said Kyrpides.
This work was supported by the ExaBiome Project, a Berkeley Lab-led collaboration which develops supercomputing tools for microbiome analysis. JGI is an Office of Science user facility.
Assessing the Costs of Major Power Outages
Little is known about the full impact of widespread, long duration power interruptions, especially the indirect costs and related economy-wide impacts of these events. As a result, the costs of such power interruptions are generally not or only incompletely considered in utility planning activities.
A new Berkeley Lab report titled "A Hybrid Approach to Estimating the Economic Value of Enhanced Power System Resilience" describes a new approach for estimating the economic costs of widespread, long duration power interruptions, such as the one that occurred recently in Texas. This hybrid method involves using survey responses from utility customers to calibrate a regional economic model that is able to estimate both the direct and indirect costs of these events.
"We believe that this paper is an important breakthrough for researchers interested in estimating the full economic impact of widespread, long duration power disruptions," said Berkeley Lab researcher Peter Larsen.
A second report, titled "Case Studies of the Economic Impacts of Power Interruptions and Damage to Electricity System Infrastructure from Extreme Events," analyzes in detail the economics of power interruptions caused by extreme weather. The researchers examined the effects of Hurricane Harvey in Texas, wildfires in California, and four other extreme events; they found that even years after the events, utility companies had a clear picture of the costs of physical repairs but had not tallied the human and societal costs of the outages. (Researchers from the University of Texas at Austin were co-authors of this report; read their news release about the report here.)
"Our research shows that utilities, regulators, and other stakeholders rarely, if ever, account for the direct and indirect costs of power disruptions in their decision-making," Larsen said.
Location, Location, Location: Regional Tau Deposits in Healthy Elders Predict Alzheimer Disease
Subtle memory deficits are common in normal aging as well as Alzheimer disease (AD), the leading cause of dementia in older adults. This makes AD difficult to diagnose in its early stages. As there is currently no effective treatment to slow or stop the progression of AD, it is important to identify early pathological brain changes, which can start decades before people show symptoms, and then trace the effects that lead to cognitive decline.
Xi Chen and her colleagues in Bill Jagust's research group at Berkeley Lab recently published a study in the Journal of Neuroscience that provides some clarification of the differences between normal aging and AD brains, and elucidates the transition from the former to the latter.
Using positron emission tomography (PET) imaging, they measured levels of tau and beta amyloid (Aß) - two critical biomarkers of AD - in cognitively normal older adults, and then followed them for several years for prospective cognition assessment. Their findings revealed new insight into tau, a protein that helps stabilize the internal skeleton of neurons in its normal form, but becomes unstable and can interfere with neuron functioning when aggregated. Aggregated tau deposits in the entorhinal cortex, a major part of the memory system, likely reflect normal, age-related memory impairment. However, tau deposits in anterior temporal regions of the brain - responsible for our knowledge of objects, people, words, and facts - were most predictive of AD-related impairment. The finding supports a model of early AD pathology proposed by the researchers whereby tau spreads from the entorhinal cortex to anterior temporal regions facilitated by amyloid.
The team suggests that the presence of tau aggregates in anterior temporal regions could be used as a marker of early disease progression.
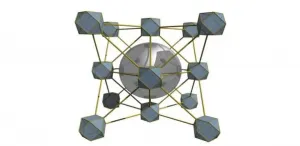
New Optical Antennas Could Overcome Data Limits
Researchers at Berkeley Lab and UC Berkeley have found a new way to harness properties of lightwaves that can radically increase the amount of data they carry. They demonstrated the emission of discrete twisting laser beams from antennas made up of concentric rings roughly equal to the diameter of a human hair, small enough to be placed on computer chips.
The new work, reported in a paper published Feb. 25 in the journal Nature Physics, throws wide open the amount of information that can be multiplexed, or simultaneously transmitted, by a coherent light source. A common example of multiplexing is the transmission of multiple telephone calls over a single wire, but there had been fundamental limits to the number of coherent twisted lightwaves that could be directly multiplexed.
"It's the first time that lasers producing twisted light have been directly multiplexed," said senior author Boubacar Kanté, a faculty scientist in Berkeley Lab's Materials Sciences Division and the Chenming Hu Associate Professor in UC Berkeley's Department of Electrical Engineering and Computer Sciences. "We've been experiencing an explosion of data in our world, and the communication channels we have now will soon be insufficient for what we need. The technology we are reporting overcomes current data capacity limits through a characteristic of light called the orbital angular momentum. It is a game-changer with applications in biological imaging, quantum cryptography, high-capacity communications and sensors."
Read the full UC Berkeley release here.
INFORMATION: