(Press-News.org) MADISON – In an advance they consider a breakthrough in computational chemistry research, University of Wisconsin–Madison chemical engineers have developed model of how catalytic reactions work at the atomic scale. This understanding could allow engineers and chemists to develop more efficient catalysts and tune industrial processes — potentially with enormous energy savings, given that 90% of the products we encounter in our lives are produced, at least partially, via catalysis.
Catalyst materials accelerate chemical reactions without undergoing changes themselves. They are critical for refining petroleum products and for manufacturing pharmaceuticals, plastics, food additives, fertilizers, green fuels, industrial chemicals and much more.
Scientists and engineers have spent decades fine-tuning catalytic reactions — yet because it’s currently impossible to directly observe those reactions at the extreme temperatures and pressures often involved in industrial-scale catalysis, they haven’t known exactly what is taking place on the nano and atomic scales. This new research helps unravel that mystery with potentially major ramifications for industry.
In fact, just three catalytic reactions — steam-methane reforming to produce hydrogen, ammonia synthesis to produce fertilizer, and methanol synthesis — use close to 10% of the world’s energy.
“If you decrease the temperatures at which you have to run these reactions by only a few degrees, there will be an enormous decrease in the energy demand that we face as humanity today,” says Manos Mavrikakis, a professor of chemical and biological engineering at UW–Madison who led the research. “By decreasing the energy needs to run all these processes, you are also decreasing their environmental footprint.”
Mavrikakis and postdoctoral researchers Lang Xu and Konstantinos G. Papanikolaou along with graduate student Lisa Je published news of their advance in the April 7, 2023 issue of the journal Science.
In their research, the UW–Madison engineers develop and use powerful modeling techniques to simulate catalytic reactions at the atomic scale. For this study, they looked at reactions involving transition metal catalysts in nanoparticle form, which include elements like platinum, palladium, rhodium, copper, nickel, and others important in industry and green energy.
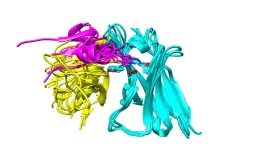
According to the current rigid-surface model of catalysis, the tightly packed atoms of transition metal catalysts provide a 2D surface that chemical reactants adhere to and participate in reactions. When enough pressure and heat or electricity is applied, the bonds between atoms in the chemical reactants break, allowing the fragments to recombine into new chemical products.
“The prevailing assumption is that these metal atoms are strongly bonded to each other and simply provide ‘landing spots’ for reactants. What everybody has assumed is that metal-metal bonds remain intact during the reactions they catalyze,” says Mavrikakis. “So here, for the first time, we asked the question, ‘Could the energy to break bonds in reactants be of similar amounts to the energy needed to disrupt bonds within the catalyst?’”
According to Mavrikakis’s modeling, the answer is yes. The energy provided for many catalytic processes to take place is enough to break bonds and allow single metal atoms (known as adatoms) to pop loose and start traveling on the surface of the catalyst. These adatoms combine into clusters, which serve as sites on the catalyst where chemical reactions can take place much easier than the original rigid surface of the catalyst.
Using a set of special calculations, the team looked at industrially important interactions of eight transition metal catalysts and 18 reactants, identifying energy levels and temperatures likely to form such small metal clusters, as well as the number of atoms in each cluster, which can also dramatically affect reaction rates.
Their experimental collaborators at the University of California, Berkeley, used atomically-resolved scanning tunneling microscopy to look at carbon monoxide adsorption on nickel (111), a stable, crystalline form of nickel useful in catalysis. Their experiments confirmed models that showed various defects in the structure of the catalyst can also influence how single metal atoms pop loose, as well as how reaction sites form.
Mavrikakis says the new framework is challenging the foundation of how researchers understand catalysis and how it takes place. It may apply to other non-metal catalysts as well, which he will investigate in future work. It is also relevant to understanding other important phenomena, including corrosion and tribology, or the interaction of surfaces in motion.
“We’re revisiting some very well-established assumptions in understanding how catalysts work and, more generally, how molecules interact with solids,” Mavrikakis says.
Manos Mavrikakis is Ernest Micek Distinguished Chair, James A. Dumesic Professor, and Vilas Distinguished Achievement Professor in Chemical and Biological Engineering at the University of Wisconsin–Madison.
Other authors include Barbara A.J. Lechner of the Technical University of Munich, and Gabor A. Somorjai and Miquel Salmeron of Lawrence Berkeley National Laboratory and the University of California, Berkeley.
The authors acknowledge support from the U.S. Department of Energy, Basic Energy Sciences, Division of Chemical Sciences, Catalysis Science Program, Grant DE-FG02-05ER15731; the Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, of the U.S. Department of Energy under contract no. DE-AC02-05CH11231, through the Structure and Dynamics of Materials Interfaces program (FWP KC31SM).
Mavrikakis acknowledges financial support from the Miller Institute at UC Berkeley through a Visiting Miller Professorship with the Department of Chemistry.
The team also used the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231 using NERSC award BES- ERCAP0022773.
Part of the computational work was carried out using supercomputing resources at the Center for Nanoscale Materials, a DOE Office of Science User Facility located at Argonne National Laboratory, supported by DOE contract DE-AC02-06CH11357.
# # #
-- Jason Daley, jgdaley@wisc.edu
END
The human body is capable of creating a vast, diverse repertoire of antibodies—the Y-shaped sniffer dogs of the immune system that can find and flag foreign invaders. Despite our ability to create a range of antibodies to target viruses, humans create antibodies that target the same viral regions again and again, according to a new study led by investigators from Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, and Harvard Medical School. These “public epitopes” mean that the generation ...
New research conducted by the University of Houston Conrad N. Hilton College of Global Hospitality Leadership suggests the Black Lives Matter movement had a significant, positive impact on the fundraising efforts of Black restaurateurs.
In a study published in the International Journal of Hospitality Management, the researchers found in the decade before Black Lives Matter rose to prominence, Black restaurateurs were 76% less likely to be successful in the use of crowdfunding sources, such as Kickstarter, than non-Black restaurateurs. From 2010 – 2015, funding was 72% lower for Black-owned businesses and from 2016-2020 it was 79% lower.
But that all changed ...
Professor Arthur Ragauskas awarded Royal Society of Chemistry prize
He is based at the Oak Ridge National Laboratory and University of Tennessee
He chose to receive award at Aston University because of its research into renewable resources.
Aston University has hosted a prestigious Royal Society of Chemistry prize-giving and lecture.
Professor Arthur Ragauskas of the Oak Ridge National Laboratory and University of Tennessee chose the University as the venue for this event because its research into renewable resources has inspired his work.
He is the recipient of the Society’s 2022 environment, sustainability and energy division open award: Environment ...
Professor Richard Hogg will be joining Aston Institute of Photonics Technologies
It is a leading photonics research centre, with a successful track record of scientific achievements
Professor Hogg will be focusing on research and commercialisation.
Aston University is to welcome Professor Richard Hogg who will be joining its flagship photonics research institute
He will be focusing on research and commercialisation at Aston Institute for Photonic Technologies, Aston University’s Aston Institute of Photonics Technologies (AIPT) a leading photonics research institute with a successful track record of ...
NASA and the James Webb Space Telescope industry team, led by Northrop Grumman Corporation, have won the prestigious National Aeronautic Association (NAA) Robert J. Collier Trophy for revolutionizing the field of astrophysics with the team’s pioneering design and exceptional performance of the telescope. The Collier Trophy is awarded annually for “the greatest achievement in aerospace and astronautics in America.” The award will be presented at the NAA Gala in Washington, D.C., on June 15.
“The James Webb Space ...
More than 125 international academics and industrials from 27 different countries will be joining Targeting Phage Therapy 2023 on June 1-2 in Paris. During Targeting Phage Therapy 2023, 50+ communications will be presented in the form of major talks, short orals, and posters.
Among the speakers
Targeting Phages 2023 will address how phages play a strategic role to combat infection and antibiotic resistance, but also to modulate gut microbiota. Phage enthusiasts and experts will be presenting their latest data and innovations.
Domenico Frezza, University of Roma Tor Vergata, Italy
“Phage Therapy: Vision, Gaps and Evolution”
Martha Clokie, ...
As the climate changes, many species are expected to adjust where and how they live. Some are expected to seek cooler elevations as it warms, but what happens to species already at the top of a mountain? A study of squirrels living in California’s high-elevation Sierra Nevada indicates that climate is only one factor to consider when trying to predict where an animal will make its home in a changing world.
The study, led by the University of California, Davis, is published in the journal Ecology and Evolution and was conducted in alpine regions stretching ...
DURHAM, N.C. – Most of us woke up this morning, used energy and technology to learn about the weather and the news, got a fresh cup of coffee, and went about our day informed and refreshed.
Imagine if every woman in a poor village in rural Africa or Asia could power on technology for vital information the same way. Yet, they cannot. Lack of energy access disempowers women.
Research demonstrates that empowered people are far more resilient to climate shocks and harms. While energy technology can advance resilience, it can also create new vulnerabilities. Think of disasters that can damage complex energy systems or destroy off-grid solar home systems.
A new review published in Nature ...
HERSHEY, Pa. — Black and Hispanic patients with severe allergies are less likely to get a common treatment, allergen immunotherapy, compared to white patients, according to Penn State College of Medicine researchers. They said identifying the causes, which could include being less likely than white patients to be referred to an allergist and the difficulty accessing treatment due to time and other resource constraints, and developing solutions for this health disparity, could help patients get relief from symptoms, including runny nose, congestion, post-nasal drip, sinus pain and ...
Firearm injury is now the leading cause of death among U.S. children and adolescents. As its toll grows, researchers have focused on stopping violence in the moments before it happens. But new research led by the University of Washington suggests that interventions made earlier in young people’s lives may reduce the chances of it happening at all.
The study, published April 6 in JAMA Network Open, found that UW’s Communities That Care (CTC) prevention system reduced handgun carrying among adolescents growing up in rural areas. By the 12th grade, adolescents in CTC communities were ...