(Press-News.org) Peer-reviewed / Randomised Controlled Trial / People
Study of healthy US adults found that a single dose of the VLA1553 vaccine candidate was generally safe, well tolerated and provokes an immune response.
After a single vaccination, the vaccine produced neutralizing antibody levels which are thought to protect against chikungunya disease in 99% (263/266) of participants.
Antibody levels declined 28 days after vaccination, but seroprotection persisted in more than 96% (233/242) participants after six months.
Most adverse events were moderate or mild and the authors say its safety profile is similar to other licensed vaccines
The VLA1553 vaccine candidate for chikungunya disease was generally well tolerated and produced an immune response in 99% (263/266) of participants, according to a phase 3 randomised controlled trial published in The Lancet.
Because the study, VLA1553-301, was not conducted in regions where chikungunya is endemic, researchers were unable to investigate whether the vaccine protects against subsequent disease. Instead, the study tested for an immune response at levels that are thought to protect against the disease if infected with the virus. [1]
Chikungunya is a mosquito-borne disease caused by the chikungunya virus (CHIKV), which is endemic in some regions of Africa, Asia, and the Americas. It causes a fever in patients roughly four to eight days after they have been bitten by an infected mosquito. Symptoms include headaches, fatigue, nausea, and severe muscle and joint pain. The joint pain is often debilitating and usually lasts for a few days but may be prolonged, lasting for weeks, months or even years. Serious disease and death is rare, but older people and newborn babies are most at risk [2]. Currently, there are no approved vaccines to prevent the disease caused by CHIKV infection nor are there effective antiviral treatments for the disease.
Lead author of the study, Dr Martina Schneider, Clinical Strategy Manager at Valneva, says, “This could be the first chikungunya vaccine available for people living in endemic regions, as well as for travellers to endemic areas or areas at risk for an upcoming outbreak. Our promising results showed good persistence of antibody levels after vaccination, which is important considering that chikungunya outbreaks may recur suddenly. As age is a risk factor for severity and mortality of chikungunya disease, the strong immune response observed in older participants might be particularly beneficial.” [3]
Study author Katrin Dubischar, Program Director, Chikungunya Vaccine at Valneva says: “At present, there is no dedicated treatment or vaccine available against chikungunya, which is a debilitating disease whose symptoms can persist on a long-term basis. Moreover, it is currently regarded as one of the viruses most likely to spread globally, and studies have shown that climate change is driving the spread of the mosquitos that carry it into new areas of the world. Therefore, having an effective vaccine is important for preparedness for future outbreaks.” [3]
The study enrolled 4,115 healthy adults across 43 study sites in the United States. 3,082 participants were given one dose of VLA1553 (via an injection in the arm), and 1,033 were given a placebo. All participants were included in the safety analysis but the immune response was only tested in a subgroup of 362 participants (266 given the vaccine and 96 given the placebo). Participants had their immune responses assessed one week, 28 days, three months, and six months after their vaccination. They also recorded adverse events in an electronic diary for 11 days after vaccination. Those who did experience adverse events within 21 days of vaccination (e.g. fever and joint pain, back pain, neurological symptoms, heart problems, rash, or swelling) were monitored more closely.
After a single vaccination, VLA1553 induced antibody levels at a level that is considered to protect against disease among 99% (263/266) of participants. There was no difference in immune response according to age.
VLA1553 was generally well tolerated across all age groups with most adverse events being mild or moderate. In those given the vaccine, the most common adverse events were headaches (experienced in 32% of vaccinated participants), fatigue (29%), muscle pain (24%), joint pain (18%), and pain at the injection site (13%).
After six months, there were more adverse events recorded for those given VLA1553 than those given placebo. Overall, 51% (1,575/3,082) of participants who were given VLA1553 and 31% (322/1,033) of those who received the placebo experienced at least one AE that was considered related to the vaccination. The safety profile in older adults was similar to that of adults.
Serious adverse events were reported in 2% (46/3,082) of participants exposed to VLA1553 and 1% of participants in the placebo arm (8/1,033). Two of these were classified as related to the vaccine. One was a case of mild muscle pain in a woman with a medical history of fibromyalgia, and the other was a fever, which resulted in hospitalisation. Neither of these cases resulted in death.
The rate of observed miscarriages in the population given VLA1553 was slightly higher than expected in the general population (23% versus around 11-16%). However, this could be due to natural variation in the small sample size. Two of the three miscarriages among women given VLA1553 were explained by genetic disorder or the participants’ history of miscarriage. In the remaining case, no reason could be identified and the authors note further monitoring will be needed as the vaccine candidate is rolled out.
Commenting on the safety outcomes of the study, Dr Juan Carlos Jaramillo, Chief Medical Officer at Valneva, says, “An independent Data Safety Monitoring Board (DSMB) evaluated safety data during the study and did not identify any safety concerns after evaluating all reported adverse events. The two related serious adverse events reported during the study both recovered fully and were reviewed by the DSMB who did not raise concerns or consider that there were serious risks caused by the vaccination in general.”
The authors note some limitations of their study. The study did not take place in an endemic region, so participants’ pre-existing immunity to the chikungunya virus is unknown, as is the safety of the vaccine in this population. In addition, the vaccine is made from a weakened version of the live virus, so is likely to be unsuitable for people with weakened immune systems, and pregnant women. They also acknowledge that to be highly effective in controlling endemic disease, a chikungunya vaccine will also need to be administered to children. To determine safety and efficacy in this age group, there is a study in adolescents in endemic areas of Brazil currently ongoing.
Writing in a linked Comment, Dr Kathryn Stephenson, Center for Virology and Vaccine Research at the Beth Israel Deaconess Medical Center, who was not involved in the study, said, “…the positive results of this trial are very good news for CHIKV pandemic preparedness. CHIKV and other arboviral infections continue to be global threats, spurred on by the expansion of mosquito habitats because of climate change and globalisation of trade and travel. Further studies of VLA1553 in endemic regions and expanded populations, such as an ongoing trial in adolescents in Brazil (NCT04650399), will be critical to affirming VLA1553’s value for CHIKV prevention, as will be real-world effectiveness studies in the context of actual CHIKV outbreaks.”
NOTES TO EDITORS
This study was funded by Valneva and Coalition for Epidemic Preparedness and Innovation. It was conducted by researchers from Valneva Austria GmbH.
[1] The FDA (US Food and Drug Administration) has deemed testing a chikungunya vaccine among adults in endemic regions who can become infected with the virus unfeasible, due to costs and logistics - https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-october-22-2020-meeting-announcement
[2] https://www.who.int/news-room/fact-sheets/detail/chikungunya , https://www.ecdc.europa.eu/en/chikungunya-monthly
[3] Quote direct from author and cannot be found in the text of the Article.
The labels have been added to this press release as part of a project run by the Academy of Medical Sciences seeking to improve the communication of evidence. For more information, please see: http://www.sciencemediacentre.org/wp-content/uploads/2018/01/AMS-press-release-labelling-system-GUIDANCE.pdf if you have any questions or feedback, please contact The Lancet press office pressoffice@lancet.com
IF YOU WISH TO PROVIDE A LINK FOR YOUR READERS, PLEASE USE THE FOLLOWING, WHICH WILL GO LIVE AT THE TIME THE EMBARGO LIFTS: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)00641-4/fulltext
END
THE LANCET: First phase 3 trial of a chikungunya vaccine candidate finds it is generally safe and provokes an immune response
2023-06-13
ELSE PRESS RELEASES FROM THIS DATE:
The chatbot will see you now:
2023-06-13
Glasgow, UK: The informed consent process in biomedical research is biased towards people who can meet with clinical study staff during the working day. For those who have the availability to have a consent conversation, the time burden can be off-putting. Professor Eric Vilain, from the Department of Paediatrics, University of California, Irvine, USA, will tell the European Society of Human Genetics annual conference today (Tuesday 13 June) how results from his team’s study of the use of a chatbot (GIA – ‘Genetics Information Assistant’ ...
NHS policies on patient’s weight and access to hip replacement surgery are inappropriate, study finds
2023-06-13
Weight and body mass index (BMI) policies introduced by NHS commissioning groups in England are inappropriate and worsening health inequalities, according to a new study published in BMC Medicine today [13 June] that analysed nearly 490,000 hip surgeries. With one in ten people likely to need a joint replacement in their lifetime, many thousands of patients are directly affected by these policies.
Rules implemented by NHS clinical commissioning groups (CCGs) across England to change the access to hip and knee replacement surgery for patients who are overweight or obese have been in effect for over ten years. ...
A baking soda solution for clean hydrogen storage
2023-06-13
In a world of continuously warmer temperatures, a growing consensus demands that energy sources have zero, or next-to-zero, carbon emissions. That means growing beyond coal, oil, and natural gas by getting more energy from renewable sources.
One of the most promising renewable energy carriers is clean hydrogen, which is produced without fossil fuels.
It’s a promising idea because the most abundant element in the universe is hydrogen, found in 75 percent of all matter. Moreover, a hydrogen molecule has two paired atoms—Gemini twins that are both non-toxic and highly combustible.
Hydrogen’s combustive potential ...
Can this medication reverse MS? Brain biomarker shows it can
2023-06-13
A decade after UC San Francisco scientists identified an over-the-counter antihistamine as a treatment for multiple sclerosis, researchers have developed an approach to measure the drug’s effectiveness in repairing the brain, making it possible to also assess future therapies for the devastating disorder.
The researchers, led by physician-scientist Ari Green, MD, who together with neuroscientist Jonah Chan, PhD, first identified clemastine as a potential MS therapy, used MRI scans to study the drug’s impact on the brain of 50 participants in a clinical study.
In MS, patients lose myelin, the protective insulation around ...
The advances and promise of continuous glucose monitoring in diabetes management
2023-06-13
As adoption of Continuous Glucose Monitoring (CGM) continues to increase, there is a growing body of evidence supporting the use of this technology in improving diabetes outcomes for patients with Type 1 or Type 2 Diabetes. A new supplement in the peer-reviewed journal Diabetes Technology & Therapeutics (DTT) provides an excellent perspective of the past, present, and future of CGM. Click here to read the supplement now.
In the supplement Rickson et al. review the rapid pace in which diabetes technology has progressed and the implications for relying on rigorous and extensive timelines to publish randomized controlled trials to impact ...
Ochsner Health names new chief financial officer and treasurer
2023-06-13
NEW ORLEANS, La. – Ochsner Health has named Jim Molloy as the organization’s next Executive Vice President, Chief Financial Officer and Treasurer. A leader at Citi bringing decades of extensive experience in healthcare finance, Molloy will oversee the organization’s accounting, financial planning and analysis, reimbursement and revenue cycle functions, as well as managed care contracting and treasury. He will also play a pivotal role in the continued development and execution of ...
Low birthweight is independently linked to increased risk of type 2 diabetes, and a particular presentation including lower age at diagnosis
2023-06-13
T2D patients with lower birthweight also show higher use of diabetes drugs than those with normal birthweight, and a larger number of comorbidities including high blood pressure, at the time of diagnosis.
The first study is by Dr Rasmus Wibaek, Steno Diabetes Center Copenhagen, Herlev, Denmark, and Dr Allan Vaag, Steno Diabetes Center Copenhagen, and also Lund University, Malmö, Sweden, and colleagues.
This study included adults aged 30–60 years enrolled in the Danish Inter99 cohort in 1999–2001 (baseline examination), with information on birthweight from original birth records from 1939–1971 and without diabetes at baseline. Birth records were linked ...
Gentle cleansers kill viruses as effectively as harsh soaps, study finds
2023-06-13
Gentle cleansers are just as effective in killing viruses – including coronavirus – as harsh soaps, according to a new study from scientists at the University of Sheffield
Healthcare professionals often substitute alcohol-based hand sanitisers and harsh soaps for skin-friendly cleansers in order to treat or prevent irritant contact dermatitis, which develops when chemical or physical agents damage the skin surface faster than the skin can repair
Incidence and severity of irritant contact dermatitis increased from 20 per cent to 80 per cent amongst healthcare professionals during the Covid-19 pandemic
Researchers also found non-enveloped ...
LP-284 targets non-Hodgkin's lymphoma and DNA damage repair deficiency
2023-06-12
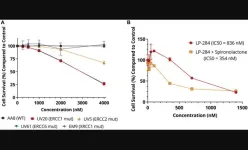
“[...] we demonstrated the new acylfulvene compound LP-284 has anti-tumor activity including nanomolar potency in fifteen in vitro NHL cell lines and in vivo preclinical NHL models.”
BUFFALO, NY- June 12, 2023 – A new research paper was published in Oncotarget's Volume 14 on June 12, 2023, entitled, “LP-284, a small molecule acylfulvene, exerts potent antitumor activity in preclinical non-Hodgkin's lymphoma models and in cells deficient in DNA damage repair.”
Despite advances in therapies treating non-Hodgkin’s ...
Damon Runyon Cancer Research Foundation announces three recipients of 2023 Physician-Scientist Training Award
2023-06-12
Three scientists with exceptional promise and novel approaches to fighting cancer have been named the 2023 recipients of the Damon Runyon Physician-Scientist Training Award. The awardees were selected through a highly competitive and rigorous process by a scientific committee comprised of leading cancer researchers who are themselves physician-scientists.
Physician-scientists are uniquely positioned to translate scientific discoveries into therapies that improve and prolong the lives of their patients. However, ...