A newly identified defect in the DOCK11 gene leads to abnormalities in both white and red blood cells. "Such rare and previously unknown diseases provide valuable insights into the fundamental principles of blood formation and the immune system. They allow us to better understand the dysregulated processes underlying these diseases," explains Kaan Boztug, senior author of the study and Scientific Director of St. Anna Children's Cancer Research Institute (St. Anna CCRI).
The gene defect was first identified in a young patient from Spain, where despite repeated tests, no explanation could be found for the severe inflammation affecting various organs such as the kidneys, intestines, and skin. The treating physician then contacted the St. Anna CCRI providing blood samples from the patient. And was successful. The internationally leading center for rare diseases of blood formation and the immune system found the cause. "We work closely with international partner institutions to understand such diseases and help the patients," says Boztug, who is also a Professor in the field of Pediatrics and Inflammation Research at the Medical University of Vienna and an Adjunct Principal Investigator at the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences.
Three additional patients with variants in the same gene
The patient's genome sequencing revealed a severe defect in the DOCK11 gene, a gene that had not been previously associated with any human disease and is involved in cell communication. "Initially, we had only one patient and therefore did not know which symptoms were related to the gene defect and which were just additional accompanying manifestations. That was quite a challenge," says Jana Block, first author of the publication and a PhD student in Boztug's research group at St. Anna CCRI and CeMM. Through their international collaborations, the researchers managed to find additional patients with similar DOCK11 mutations, which helped provide a clearer understanding of the disease.
DOCK11 controls blood formation
One of the patients suffered from a significantly reduced number of red blood cells and required regular blood transfusions. "Anemia often occurs with immune deficiencies, and often, a person's own red blood cells are destroyed by autoantibodies that target their own blood cells. In healthy people, however, this does not happen,” says Jana Block. “However, in our investigations, we did not find such autoantibodies." The cause of the decreased number of red blood cells was clarified by the researchers using a zebrafish model. The DOCK11 defect resulted in impaired blood cell formation, leading to a novel mechanism for anemia, a deficiency of red blood cells.
DOCK11 keeps T cells in check
The role of DOCK11 in humans was, until now, unexplored; previous studies had shown the importance of the protein for the development of B cells in mouse models. The researchers have now shown that to a certain extent B cells also do not develop properly in humans with DOCK11 deficiency, and at the same time, other immune cells, called T lymphocytes, are overactivated. "The protein appears to play a role in keeping the activation of T cells within a certain range," explains Block. This is important because overactivation can lead to damage to surrounding tissues and organs, especially in the absence of an actual pathogen. A connection between T-cell defects and predisposition to tumor diseases is observed in other immune deficiencies and cannot be ruled out in the case of DOCK11 deficiency. "We will certainly investigate this question further in our research," says Boztug.
Stem cell transplantation as a possible treatment
Although not all details of DOCK11's function are yet understood, the researchers suspect that transplantation of blood-forming stem cells could cure the disease. "Of course, these questions need to be investigated in future studies. It is also conceivable that DOCK11 deficiency could be treated through gene therapy," says Boztug. The fact that there is now hope for the development of therapies has a very special significance for the scientists: "Our work over the past five years is undoubtedly a significant milestone, but we are also aware that several of the patients have died from the disease," says Block. The researcher hopes that now other centers will become aware of this gene mutation and further advance the exploration of this disease. "This disease is truly severe, so we see it as our mission not only to understand the biological processes but also to develop effective therapeutic strategies based on that," adds Boztug.
--
Fotos
Univ.-Prof. Dr. Kaan Boztug
Credit: Ian Ehm
Jana Block, MSc
Credit: St. Anna Kinderkrebsforschung
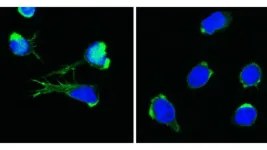
Gesunde und DOCK11-defiziente T Zellen mit sichtbarem Zellkern (blau) und Aktin-Zytoskelett (grün)
Credit: St. Anna Kinderkrebsforschung
Publication
Systemic inflammation and normocytic anemia in DOCK11 deficiency
Jana Block, Christina Rashkova*, Irinka Castanon*, Samaneh Zoghi, Jessica Platon, Rico Chandra Ardy, Mitsuhiro Fujiwara, Beatriz Chaves, Rouven Schoppmeyer, Caspar I. van der Made, Raul Jimenez Heredia, Frederike Leonie Harms, Samin Alavi, Laia Alsina, Paula Sanchez Moreno, Rainiero Avila Polo, Rocio Cabrera-Perez, Sevgi Kostel Bal, Laurene Pfajfer, Bernhard Ransmayr, Anna-Katharina Mautner, Ryohei Kondo, Anna Tinnacher, Michael Caldera, Michael Schuster, Cecilia Dominguez Conde, Rene Platzer, Elisabeth Salzer, Thomas Boyer, Han G. Brunner, Judith E. Nooitgedagt-Frons, Estibaliz Iglesias Jimenez, Angela Deya-Martinez, Marisol Camacho Lovillo, Jorg Menche, Christoph Bock, Johannes B. Huppa, Winfried F. Pickl, Martin Distel, Jeffrey A. Yoder, David Traver, Karin R. Engelhardt, Tobias Linden, Leo Kager, J. Thomas Hannich, Alexander Hoischen, Sophie Hambleton, Sabine Illsinger, Lydie Da Costa, Kerstin Kutsche, Zahra Chavoshzadeh, Jaap D. van Buul, Jordi Anton, Joan Calzada-Hernandez, Olaf Neth, Julien Viaud, Akihiko Nishikimi, Loic Dupre, Kaan Boztug
*, contributed equally to this work
New England Journal of Medicine, June 21, 2023
DOI: 10.1056/NEJMoa2210054
Funding
The study was funded by: European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (820074 an K.B.), dem Vienna Science and Technology Fund (WWTF-LS16-060 to K.B., L.D. and J.M.), die Deutsche Forschungsgemeinschaft (KU 1240/13-1 to K.K.), DOC fellowships of the Austrian Academy of Sciences (5590 to J.B. and 24486 to R.C.A.), CNRS (IRP program, SysTact project to L.D.) and CAPES PRINT (to B.C.) grants, Wellcome Trust (207556/Z/17/Z to S.H.), the ZonMW NWO Vici grant (91819632 to J.D.v.B. and R.S.), the Austrian Research Promotion Agency (FFG) project (7940628 Danio4Can to M.D.), a German Academic Exchange Service (DAAD) postdoctoral fellowship and an EMBO fellowship (to M.D.), and by research funding for longevity sciences from the National Center for Geriatrics and Gerontology (21-27-2 to A.N.).
About Kaan Boztug
Univ.-Prof. Dr. Kaan Boztug is the Scientific Director of St. Anna Children's Cancer Research Institute, Senior Physician in Pediatric Hematology and Oncology, Head of the Immunology Department at St. Anna Children's Hospital, and Professor in the Department of Pediatrics and Adolescent Medicine at the Medical University of Vienna. He is an internationally recognized expert in rare diseases of blood formation and the immune system, and a two-time recipient of the ERC Grant (ERC Starting and Consolidator Grant). He has been honored with numerous awards for his scientific work. Under his leadership, scientific papers have been published in top journals such as the New England Journal of Medicine, Blood, Nature Immunology, and Nature Genetics. Kaan Boztug's research group focuses on inherited bone marrow failure syndromes, immune deficiencies, and inherited predisposition to childhood tumors. Their aim is to understand fundamental mechanisms of immune surveillance that are relevant to pediatric oncology and immunotherapy approaches.
After studying medicine in Düsseldorf, Freiburg, and London, and completing his doctoral studies at the Scripps Research Institute in La Jolla, USA, Kaan Boztug underwent clinical training and postdoctoral research at the Hannover Medical School. Since 2011, he has been working as a physician and researcher at the Medical University of Vienna in the Department of Pediatrics and Adolescent Medicine, as well as an Adjunct Principal Investigator at CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences. In addition, Kaan Boztug is also the Director of the CeRUD Vienna Center for Rare and Undiagnosed Diseases at the Medical University of Vienna and has been the Director of the Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases.
About St. Anna Children's Cancer Research Institute
St. Anna Children's Cancer Research Institute (CCRI) is an international and interdisciplinary research institution dedicated to advancing diagnostic, prognostic, and therapeutic strategies for the treatment of children and adolescents with cancer through innovative research. Incorporating the specific characteristics of childhood tumor diseases, dedicated research groups collaborate in the fields of tumor genomics and epigenomics, immunology, molecular biology, cell biology, bioinformatics, and clinical research. Their aim is to bridge the latest scientific and experimental knowledge with the clinical needs of physicians in order to significantly improve the well-being of young patients. For more information, visit www.ccri.at or www.kinderkrebsforschung.at.
About the Medical University of Vienna
The Medical University of Vienna (MedUni Vienna) is one of the most prestigious institutions for medical education and research in Europe. With approximately 8,000 students, it is the largest medical training institution in the German-speaking region. With 6,000 employees, 30 university clinics, two clinical institutes, 13 medical-theoretical centers, and numerous highly specialized laboratories, it is considered one of the most significant biomedical research institutions in Europe. The MedUni Vienna also houses the Josephinum, a museum of medical history. For more information, visit www.meduniwien.ac.at.
About the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences (ÖAW)
The CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences is an international, independent, and interdisciplinary research institution for molecular medicine under the scientific leadership of Giulio Superti-Furga. CeMM is guided by medical needs and integrates basic research with clinical expertise to develop innovative diagnostic and therapeutic approaches for precision medicine. The research focuses on cancer, inflammation, metabolic and immune disorders, rare diseases, and cellular aging processes. The research facility is located on the campus of the Medical University of Vienna and the General Hospital Vienna. For more information, visit www.cemm.at
END