The herpes simplex virus-1 can sometimes cause a dangerous brain infection. Combining an anti-inflammatory and an antiviral could help in these cases, report scientists with the Rajewsky and Landthaler labs and the Organoid Platform at the Max Delbrück Center in Nature Microbiology.
About 3.7 billion people — 67% of us — carry the herpes simplex virus-1 in our nerves cells where it lies quiescent until triggered by stress or injury. When activated, its symptoms are usually mild, limited to cold sores or ulcers in our mouth.
Very rarely, the virus can travel up the neurons to the brain, where it can cause a life-threatening infection. This accounts for 5 to 15% of all cases of infectious encephalitis in children and adults. Doctors typically prescribe an anti-viral called acyclovir. But even so, the patients often suffer from long-lasting and debilitating memory loss, seizures and other cognitive disorders.
In such cases, doctors could trial an anti-viral in combination with a drug that curbs inflammation to see whether it offers a better prognosis, suggests a new “Nature Microbiology” study by scientists at the Max Delbrück Centre for Molecular Medicine in the Helmholtz Association in Berlin. The scientists made this discovery using a three-dimensional model of the brain grown from human stem cells. The use of such models, called organoids, is at the frontier of clinical medicine.
“These proto-brains contain hundreds of thousands of neurons that can communicate with each other in a synchronized manner. Important experiments can be conducted with them that were impossible a few years ago,” says Professor Nikolaus Rajewsky, Scientific Director of the Berlin Institute for Medical Systems Biology at the Max Delbrück Center (MDC-BIMSB) and senior author of the study.
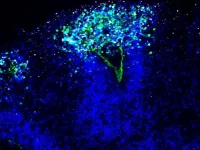
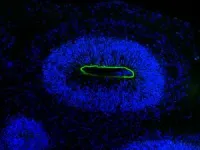
Dr. Agnieszka Rybak-Wolf, who heads the Organoid Technology Platform at the Max Delbrück Center and is one of the first authors, created the organoids, which were white, 0.5 cm blobs. “Brain organoids look a bit like small clouds of tissue,” she says.
Closer to reality for herpes
Without organoids, analyzing HSV-1-induced encephalitis is challenging. The virus infects only people and getting these brain samples is impractical. Scientists defaulted to studying the disease in cultured nerve cells or in mice, which are not natural carriers of the virus.
“This model is now much closer to reality for the herpes virus than what has been used previously,” says Dr. Emanuel Wyler, a virus expert who studies the molecular mechanisms of HSV-1 infections at the Landthaler lab and one of the first authors.
The scientists infected the organoids with the HSV-1 virus and visualized the neuroepithelial and neuronal cells as the virus rampaged and the mini-brain disintegrated. “We had these beautiful microscopy images that are so clear and you can see what is actually going on,” Wyler says.
They next conducted a single cell analysis to identify all the molecular pathways active during infection. “We used an unbiased approach to find all the pathways and genes that matter,” says Dr. Ivano Legnini, a systems biologist previously at the Rajewsky lab, and one of the first authors. “We bring systems biology to the table.”
They noticed that a signaling pathway important in inflammation, called TNF-α, was highly active. When they treated the organoids with acyclovir, the standard of care for HSV-1 encephalitis, viral replication stopped – but the tissue damage continued. Further analysis showed the TNF-α pathway was still active despite treatment.
A defense that can become damaging
“The inflammation pathway is a key natural defense to the virus,” says Dr. Tancredi Massimo Pentimalli, a medical doctor now doing his PhD in systems medicine at the Rajewsky lab and one of the first authors. “But when we block viral replication with anti-viral drugs, the overzealous inflammatory response could instead become damaging.”
Rybak-Wolf treated the organoids with both an anti-viral and an anti-inflammatory drug, which would turn off the TNF-α pathway. This combined treatment prevented the damage of mini-brains. “There is a signaling pathway in the brain that becomes active during infection,” she says. “When we switched it off using these drugs, the organoid wasn’t damaged.”
The scientists hope doctors will trial acyclovir and an anti-inflammatory as a treatment for HSV-1 encephalitis. “I hope that clinical investigators will set up clinical trials evaluating the efficacy of new anti-viral and anti-inflammatory combination therapies in herpes encephalitis patients, ultimately translating our findings from the bench to the bed side,” Pentimalli says.
Max Delbrück Center
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (Max Delbrück Center) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the locations in Berlin-Buch and Mitte, researchers from some 70 countries study human biology – investigating the foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium of a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The Max Delbrück Center therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the Max Delbrück Center today employs 1,800 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin.
END