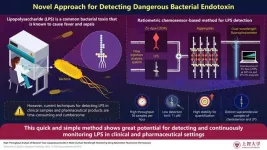
The COVID-19 pandemic made it very clear that we need better methods to quickly screen for dangerous pathogens and substances. One such compound that regularly flies under the radar is lipopolysaccharide (LPS), largely known as "endotoxins." This molecule, which is found in the outer membrane of Gram-negative bacteria, can be very harmful to humans. It can trigger a major immune response, producing fever and inflammation. In the worst cases, it can cause organ failure due to sepsis.
Surprisingly, for such a ubiquitously present toxin, there are very few ways to effectively detect the presence of LPS. The gold standard for its detection is the limulus amebocyte lysate (LAL) test. Because this has to be done manually in a clean laboratory setting, the procedure can take several hours and is also expensive. While there are other methods to detect LPS, they too are time-consuming or cumbersome. And the time taken here can sometimes cause significant delays in decision-making at hospitals and pharmaceutical manufacturing sites.
Against this backdrop, a research team from Japan have pioneered a new strategy to quickly detect LPS in soluble samples. In their latest study, published online in the journal Analytical Chemistry on July 31, 2023, the team presents a promising platform that could revolutionize how we screen for LPS. The first author of the study is Hiroshi Kimoto, who is a PhD student at Sophia University, Japan, and a member of the Technical Development Division at Nomura Micro Science Co., Ltd. The study was co-authored by Takashi Hayashita and Takeshi Hashimoto, both from Sophia University, and Yota Suzuki from Saitama University.
The main component of the proposed LPS analysis system is a ratiometric fluorescent chemosensor called Zn-dpa-C2OPy. This compound, which was designed to bind selectively to LPS, exhibits interesting fluorescent properties. When not bound to LPS, it forms small spherical vesicles that emit light with a certain wavelength when excited by UV rays. However, in the presence of LPS, the chemosensor forms complex aggregates with the toxin in a solution; these aggregates are structurally distinct from the aggregates of either the chemosensor or LPS alone. The complex chemosensor-LPS aggregates emit light at a completely different wavelength when excited by UV rays, and their presence was further verified via spectrometric measurements.
To achieve high-throughput LPS detection, the researchers combined the chemosensor with a flow injection analysis (FIA) system and a self-developed dual-wavelength fluorophotometer. This system allows one to easily mix a liquid sample of interest with a known quantity of chemosensor, and the mixture is then fed into the fluorophotometer, which measures the fluorescence changes in response to LPS. Based on the ratio between the fluorescence intensities, one can estimate the concentration of LPS in the input sample. One of the main benefits of this system is its speed. “It takes only one minute from sample collection to analysis results, with an hourly sample throughput of 36, making this technique extremely rapid and efficient,” remarks Kimoto.
In addition to the high throughput, the proposed chemosensor exhibits high sensitivity and stability to quantify LPS. In fact, the chemosensor has a detection limit of 11 pM (picomolar), which is lower than that of other reported small molecule chemosensors for LPS detection. This means that it can detect lower concentrations of LPS than other alternative methods can. Moreover, the chemosensor-based analysis system is simple and animal-friendly—other conventional LPS detection methods use animal resources and may, in turn, harm these animals. This makes it a great candidate for practical and efficient point-of care testing for LPS and bacterial contaminations in water, clinical, or pharmaceutical samples.
Envisioning the long-term implications of this work, Mr. Kimoto comments, “Based on this research, an online-endotoxin monitor will be developed and made available for use in real-life situations. Such a monitor could be installed at pharmaceutical production sites, hospital bedsides, and intensive care units to continuously monitor endotoxin concentration in pharmaceutical products, such as water for injection, or the blood of infected patients.”
With more work in the field, the threat of endotoxins will be minimized in the near future, making hospitals safer and improving diagnostic procedures for bacterial diseases.
Reference
【Title of original paper】High-Throughput Analysis of Bacterial Toxic Lipopolysaccharide in Water by Dual-Wavelength Monitoring Using Ratiometric Fluorescent Chemosensor
【Journal】Analytical Chemistry
【DOI】10.1021/acs.analchem.3c01870
【Authors】Hiroshi Kimoto1,2, Moeka Takahashi3, Masakage Masuko1, Kai Sato1, Yuya Hirahara1,2, Masamitsu Iiyama2, Yota Suzuki3,4, Takeshi Hashimoto3, and Takashi Hayashita3*
【Affiliations】1Graduate School of Science and Technology, Sophia University, Japan, 2Technical Development Division, Nomura Micro Science Co., Ltd., Japan, 3Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, Japan, 4Graduate School of Science and Engineering, Saitama University, Japan
About Sophia University
Established as a private Jesuit affiliated university in 1913, Sophia University is one of the most prestigious universities located in the heart of Tokyo, Japan. Imparting education through 29 departments in 9 faculties and 25 majors in 10 graduate schools, Sophia hosts more than 13,000 students from around the world.
Conceived with the spirit of “For Others, With Others,” Sophia University truly values internationality and neighborliness, and believes in education and research that go beyond national, linguistic, and academic boundaries. Sophia emphasizes on the need for multidisciplinary and fusion research to find solutions for the most pressing global issues like climate change, poverty, conflict, and violence. Over the course of the last century, Sophia has made dedicated efforts to hone future-ready graduates who can contribute their talents and learnings for the benefit of others, and pave the way for a sustainable future while “Bringing the World Together.”
Website: https://www.sophia.ac.jp/eng/
About Hiroshi Kimoto from Sophia University
Hiroshi Kimoto is working towards his PhD degree at the Graduate School of Science and Technology at Sophia University, Japan. He is also affiliated with the Technical Development Division of Nomura Micro Science Co., Ltd. He specializes in chemosensors, spectrometric analysis, and flow analysis. He currently has eight publications to his name on these topics.
Funding information
This study was funded by a Grant-in-Aid for Scientific Research (C) (grant no. 23K04792), a Grant-in-Aid for Scientific Research (B) (grant no. 20H02772), and a JSPS Research Fellowship for Young Scientists PD (grant no. 21J00709 and 22KJ2748) from the Japan Society for the Promotion of Science (JSPS). This work was also supported by Sophia University Special Grant for Academic Research; “Adopted Research Projects of Research on Optional Subjects”.
END