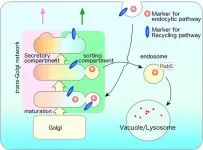
Endocytosis is an important cellular process through which cells internalize substances such as water and nutrients. These substances are first transported as cargo to the initial sorting compartment (endosomes) before being degraded (endo-lysosomal pathway) or recycled (recycling pathway of the plasma membrane). The trans-Golgi network (TGN), which lies adjacent to the Golgi apparatus, is a key mediator of this intracellular transport. Endocytosis mediates the infection of harmful pathogens such as bacteria and virus, and its disruption may lead to several diseases. It is, therefore, crucial to understand the molecular mechanisms that govern this process. Budding yeast are an important model for studying endocytosis, but the specific mechanisms of endocytic cargo sorting in these organisms have long remained unclear.
A study published on July 21, 2023, in the international journal eLife, has uncovered a new endocytic pathway in budding yeast. The findings demonstrate that a specific compartment within the TGN — marked by a protein called Tlg2p — serves as an early compartment and sorts endocytic cargos for either recycling or degradation. These exciting findings are the result of a major international collaboration between a large group of eminent researchers, including Prof. Jiro Toshima of the Department of Biological Science and Technology, Tokyo University of Science, Prof. Junko Y Toshima of the School of Health Science, Tokyo University of Technology, Prof. Daria E Siekhaus of the Institute of Science and Technology, Austria, and Dr. Akihiko Nakano of RIKEN's Center for Advanced Photonics. “Endocytic sorting mechanisms differ across different types of organisms, like plants and animals. Our findings answer the long-standing question of whether yeasts, which are common model organisms for endocytosis, possess a specific sorting compartment early in the endocytic pathway,” explains Prof. Toshima.
A key tool for this discovery was a fluorescent endocytosis marker, which was previously developed by Prof. Toshima and his colleagues. In combination with high-speed 4D imaging using highly sensitive super-resolution confocal live imaging microscopy (SCLIM), developed by Dr. Nakano’s group (RIKEN, Wako), this tool allowed the researchers to observe the dynamics of endocytic cargo. Microstructural studies using this approach showed that after endocytosis, the fluorescent endocytosis marker was first taken up by a specific, independent compartment in the TGN. Importantly, this compartment contained Tlg2p, which is a target protein for endocytic vesicle.
Notably, both substances and proteins destined for degradation by lysosomes and recycling by the plasma membrane were found to first localize to the same Tlg2p compartment. These findings suggest that this sorting compartment sends endocytic cargo materials to lysosomes for degradation and to the plasma membrane for recycling. The team also found that the Golgi-associated, γ-adaptin ear containing, Arf-binding (GGA) proteins were required as adaptors for this sorting mechanism.
Further analysis demonstrated that the Tlg2p-residing compartment was spatially distinct from the secretion compartment within the same TGN, suggesting its independent existence within the cell. In addition, while the deletion of GGA affected sorting dynamics in the Tlg2p-residing compartment, it had no effect on the secretion compartment. Together, these findings indicated that different TGN compartments may be involved in secretion and endocytic sorting.
The new endocytic mechanisms identified in this study are suggestive of similar transport pathways (compartments) in mammalian cells. Hence, they may hold the key to uncovering the molecular mechanisms of basic life processes as well as the mechanisms of infections and diseases caused by disruptions in endocytosis. Prof. Toshima elaborates, “Our findings have potential for clinical translation. In the future, they could be used to develop therapeutic strategies to prevent/treat pathogens infections and other diseases caused by endocytic disorders. This will be even more significant given the emergence of new pathogens, which is only expected to increase in the future.”
***
Reference
DOI: https://doi.org/10.7554/eLife.84850
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society", TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Professor Jiro Toshima from Tokyo University of Science
Dr. Jiro Toshima is a Professor in the Department of Biological Science and Technology at the Tokyo University of Science, Japan. He is a renowned cell biologist and received his doctoral degree from Kyushu University in 1999. His research focuses on endocytosis and the actin cytoskeleton, and he has over 45 publications to his credit. Since September 2017, he has served as the Councilor of the Japanese Biochemical Society.
Funding information
This work was supported by the Japan Society for the Promotion of Science (#18K062291 for Junko Y Toshima; #19K065710 for Jiro Toshima), Takeda Science Foundation for Junko Y Toshima and Jiro Toshima, and Life Science Foundation of Japan for Jiro Toshima.
END