Type of work: peer-reviewed/clinical trial/people
A major clinical trial shows that the drug, esketamine, one of the two main forms of ketamine, outperforms one of the standard treatments for treatment-resistant major depression. This industry-funded work is presented for the first time at the 36th ECNP Congress in Barcelona, with publication in the peer-reviewed journal the New England Journal of Medicine (see Notes for details).
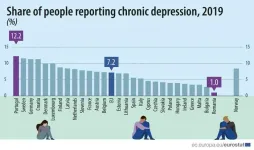
Clinical depression (also known as MDD, major depressive disorder) affects a significant number of people at any one time, giving them real life problems and increasing the health-related costs. Eurostat reports that 7% of the EU adult population had problems with depression in 2019, and around 20% to 30% of these patients do not respond to treatment. If these patients do not respond after two consecutive treatments, then they are classified as having treatment-resistant depression. Almost all MDD patients in hospital care suffer from treatment-resistant depression.
The antipsychotic drug quetiapine is commonly used in treatment-resistant depression (normally used together with an antidepressant). However, esketamine NS is the only specifically approved therapy for treatment-resistant depression in Europe (it is also given alongside other antidepressants). Esketamine NS is a nasal spray, approved in 2019.
Now the ESCAPE-TRD study describes the first large trial comparing esketamine with quetiapine. This study was funded by Janssen EMEA, the manufacturers of esketamine NS.
Researcher Andreas Reif (of Goethe University Frankfurt), first author of the study, said “The ESCAPE-TRD trial was an open-label, single-blind, randomised, controlled trial, conducted across 171 sites comprising hospitals, inpatient and outpatient clinics, and research centres in 24 countries. This is the first trial to compare this new treatment with a standard existing treatment for treatment-resistant depression, and so it’s a really necessary study. The results are very positive”.
The patients were aged between 18 and 74. All had treatment-resistant depression, in some cases the depression persisted after six different treatment attempts. All had been taking antidepressants, such as SSRI (selective serotonin reuptake inhibitor) or SNRI (serotonin and norepinephrine reuptake inhibitors). 336 patients then received esketamine nasal spray plus an SSRI or SNRI, while another 340 patients received quetiapine plus an SSRI or SNRI. Patients were treated for eight weeks, followed by 24 weeks of maintenance treatment.
Professor Reif continued, “We were testing patients at two endpoints (goals). The first major endpoint was to understand the proportion of patients who achieved remission after eight weeks. The second was determining the proportion of patients who met the first endpoint and who were relapse free at the end of the trial period (i.e. after 32 weeks). We measured the effects of treatment using a standard depression scale, the Montgomery‑Åsberg Depression Rating Scale”.
Results
After eight weeks, 28% patients taking esketamine plus antidepressants achieved remission, as against 18% remission in the group taking quetiapine (primary endpoint). At the 32-week mark (key secondary endpoint), 22% of patients taking it were still in remission, as opposed to 14% of patients who had taken quetiapine plus antidepressants.
“There were other differences we saw over time”, said researcher Professor Allan Young (of Kings College London who collaborated with the study). “For example the patients receiving the esketamine treatment had fewer depressive symptoms than those taking quetiapine. We found that patients receiving esketamine NS were around 1.5 times as likely to experience remission at Week 8 than those receiving quetiapine XR. In addition, esketamine NS‑treated patients were 1.5 times as likely to achieve the key secondary endpoint, remaining relapse free through Week 32. Indeed, by Week 32, approximately half of patients receiving esketamine NS were in remission, while two thirds were responders, emphasising the importance of continuing treatment in those who do not achieve remission in the acute phase”.
Commenting, Dr Josep Antoni Ramos-Quiroga from Vall Hebron University Hospital (CIBERSAM) and Autonomous University of Barcelona said: “The results of this study show the superior response and safety of esketamine nasal spray when compared with quetiapine. This gives people with treatment-resistant depression more safe treatment options".
This is an independent comment, Dr Ramos-Quiroga was not involved in this work.
Notes
Funding: This study was funded by Janssen EMEA, manufacturers of esketamine NS.
This press release is developed from work presented at the 36th ECNP Congress in Barcelona, 7-10 October 2023 (listed below). All press releases from the ECNP congress are produced independently of any sponsorship or external interest.
ECNP Presentation
Andreas Reif will present this work during the ECNP New Medications symposium on 8 October, https://www.ecnp.eu/congress2023/ECNPcongress/programme/programme#!sessiondetails/0000122610_0
Relevant Posters from the ECNP Congress, presented Sunday 8 October.
P.0149 Duration and impact of adverse events with esketamine nasal spray and quetiapine extended release in the ESCAPE‑TRD phase IIIb trial E. Vieta1, P.M. Llorca2, A.J. Oliveira-Maia3,4, L. Häggström5, W. Cubała6, J. Buyze7, Y. Godinov8, B. Rive9, A. Reif10
P.0146 Remission/response with esketamine nasal spray versus quetiapine extended release in treatment resistant depression using the Clinical Global Impression‑Severity scale A. Reif1, A. Fagiolini2, A.J. Oliveira-Maia3,4, A. Luts5, N. Cardoner6, J. Buyze7, S. Mulhern-Haughey8, Y. Kambarov7, A.H. Young9,10
This work is presented at the 36th ECNP Congress, which takes place in Barcelona and online on 7-10 October 2023, see https://www.ecnp.eu/Congress2023/ECNPcongress. With more than 5,300 participants the ECNP Congress is Europe’s leading platform for the latest research in disease-related neuroscience.
This work is also published on 5 October, in the New England Journal of Medicine. “Esketamine Nasal Spray versus Quetiapine for Treatment-Resistant Depression”: Andreas Reif et al. DOI: 10.1056/NEJMoa2304145 Embargo as indicated above.
END