(Press-News.org) People have only 20,000 to 30,000 genes (the number is hotly contested), but they use those genes to make more than 2 million proteins. It's the protein molecules that domost of the work in the human cell. After all, the word protein comes from the Greek prota, meaning "of primary importance."
Proteins are created as chains of amino acids, and these chains of usually fold spontaneously into what is called their "native form" in milliseconds or a few seconds.
A protein's function depends sensitively on its shape. For example, enzymes and the molecules they alter are often described as fitting together like a lock and key. By the same token, misfolded proteins are behind some of the most dreaded neurodegenerative diseases, such as Alzheimer's, Parkinson's and mad cow disease.
Scientists can't match the speed with which proteins fold. Predicting how chains of amino acids will fold from scratch requires either powerful supercomputers or cloud sourcing (harnessing the pattern recognition power of thousands of people by means of games such as Folding@home).
Either way, prediction is time-consuming and often inaccurate, so much so that the protein-structure bottleneck is slowing the exploitation of DNA sequence data in medicine and biotechnology.
A clever way of watching proteins fold and unfold may finally provide the kind of detail needed to improve protein structure predictions.
In a recent issue of the online version of the Journal of the American Chemical Society three scientists, led by Michael L. Gross, PhD, professor of chemistry in Arts & Sciences and of medicine and immunology in the School of Medicine at Washington University in St. Louis, describe a proof-of-principle study in which they use the new approach to watch the folding of a small protein called barstar.
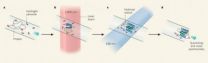
What they do is roughly analogous to filming flying bullets or bursing balloons with a stroboscope and a fast camera. The "stills" taken by the camera slow motion to the point that normally imperceptible events are laid open to scrutiny.
The scientists are using a version of this old trick to "watch" proteins fold. The "strobe light" is a temperature jump and the "camera" is a fast chemical reaction whose outcome is measured by a sensitive mass spectrometer.
Why folding is a complex problem
One of the dogmas of modern biology is that the sequence of amino acids determines how a protein will fold. If the amino acid sequence is known, it should be possible to calculate the protein's final structure from scratch.
But like many things in life, it's harder than it looks.
"Think of a protein as thousands of atoms connected together by springs," says Gross, who is also director of the National Institutes of Health/ National Center for Research Resources (NIH/NCRR) Mass Spectrometry Resource "If you were to suspend this object with a string from the ceiling and let it flop around, imagine how many shapes it could take."
"An enormous number, because it is free to move in so many different ways."
In practice, scientists often predict protein structure not from scratch but by analogy. They sift through large databases for proteins with similar sequences of amino acids and assume similar amino-acid chains will fold in similar ways.
"But," says Gross, "at some point any method for predicting protein structure has to be checked against experimental evidence that shows how proteins actually do fold."
That's what his research is all about.
A model protein for the experiment
Barstar is a small protein synthesized by a soil bacterium that is often used in folding studies.
Importantly, barstar's "native state" is known, as is its primary structure, the sequence of the protein subunits called amino acids of which it is made. What isn't known is how the amino-acid chain twists and coils to form the final structure.
Fortunately for the scientists, barstar, unlike most proteins, is unfolded at zero degrees Celsius and begins to fold as it warms.
The folding takes place in microseconds (thousandths of a second).
How the method works
The scientists begin by injecting a cold solution of barstar and a tiny amount of hydrogen peroxide into an optical fiber so thin it is difficult to believe it is actually hollow.
"Plugs" of sample in the fiber are then hit with two laser pulses in quick succession.
The first pulse, called a T jump, heats the solution just enough to make a different protein conformation energetically favorable.
The second pulse then breaks some of the hydrogen peroxide (H2O2) molecules into two haves, each of which is a very reactive hydroxyl (-OH) radical.
The radicals react with those parts of the protein that are exposed to the solution, "painting" them with oxygen atoms.
"Imagine," says Gross, "that you suspended a styrofoam model of a partially folded protein and spray-painted it blue. The outside parts would be painted blue; those buried within would remain white."
The radical reactions must be terminated rapidly; otherwise some "painting" may occur within the structure. Within a microsecond, a scavenger amino acid clears away any remaining hydroxyl radicals to prevent them from breaking bonds and altering the protein's configuration.
The same process is repeated 500 times, taking rapid-fire "snapshots" of the protein's quickly changing confirmation.
"The hydroxyl radicals don't mark everything," says Gross. "But they mark about half the amino acids, which is really pretty good. Most other chemical reagents are too specific and too slow for this experiment. Compared to hydroxyl radicals they're just plain ponderous."
Weighing the painted proteins
"We collect each drop of marked protein as it emerges from the fiber," says Gross. "Then we digest the protein very slowly and carefully with an enzyme that cleaves the amino acid chains at specific locations, creating a known set of protein fragments, called peptides
These protein fragments are separated according to type by liquid chromatography, and a mass spectrometer then "weighs" each fragment type to see whether it has picked up oxygen atoms.
"Detecting an extra oxygen is child's play for a modern mass spectrometer," says Gross. "Most instruments can even detect an extra proton, with is one-sixteenth the mass of an oxygen atom."
"In the same instrument, on the fly, we break apart the protein fragments and again 'weigh' the bits to see which one still carries the oxygen atom. This lets us deduce the oxygen's location on the original fragment."
By following barstar to its first intermediate state, or way station enroute to its native state, the scientists demonstrated that the new technique can follow folding and unfolding on a submillisecond time scale.
'Massive amounts of detail'
Gross is the first to say that this proof-of-principle experiment stands at the end of a long line of elegant experiments of a similar type, called pump-probe experiments.
Other techniques probe the change in protein structure by monitoring the absorption or emission of light--or a similar physical effect. They can provide only global information, such as the rate constant of a folding reaction.
"Because we use a chemical rather than a physical probe, we can see what's going on in much greater detail," says Gross. "We can say which part of the structure closes first, which second, and so on."
The new technique caught the attention of protein scientist Martin Gruebele of the University of Illinois, who spotlighted it in the Dec. 2, 2010, issue of the journal Nature.
It "could provide truly massive amounts of detail about fast protein folding," wrote Gruebele, which might finally allow scientists "to correctly predict the biologically active structure of a protein starting from the unfolded state."
INFORMATION:
New method takes snapshots of proteins as they fold
Scientists have invented a way to 'watch' proteins fold -- in less than thousandths of a second -- into the elaborate twisted shapes that determine their function
2011-01-11
ELSE PRESS RELEASES FROM THIS DATE:
Universities miss chance to identify depressed students
2011-01-11
CHICAGO --- One out of every four or five students who visits a university health center for a routine cold or sore throat turns out to be depressed, but most centers miss the opportunity to identify these students because they don't screen for depression, according to new Northwestern Medicine research.
About 2 to 3 percent of these depressed students have had suicidal thoughts or are considering suicide, the study found.
"Depression screening is easy to do, we know it works, and it can save lives," said Michael Fleming, professor of family and community medicine ...
'Hot-bunking' bacterium recycles iron to boost ocean metabolism
2011-01-11
In the vast ocean where an essential nutrient—iron—is scarce, a marine bacterium that launches the ocean food web survives by using a remarkable biochemical trick: It recycles iron.
By day, it uses iron in enzymes for photosynthesis to make carbohydrates; then by night, it appears to reuse the same iron in different enzymes to produce organic nitrogen for proteins.
The bacterium, Crocosphaera watsonii, is one of the few marine microbes that can convert nitrogen gas into organic nitrogen, which (just as it does on land) acts as fertilizer to stimulate plant growth in ...
Men with macho faces attractive to fertile women, researchers find
2011-01-11
When their romantic partners are not quintessentially masculine, women in their fertile phase are more likely to fantasize about masculine-looking men than are women paired with George Clooney types.
But women with masculine-looking partners do not necessarily become more attracted to their partners, a recent study co-authored by a University of Colorado at Boulder researcher concludes.
Meanwhile, a man's intelligence has no effect on the extent to which fertile, female partners fantasize about others, the researchers found. They say the lack of an observed "fertility ...
GEN reports on biotech acquisition deals in 2010 that topped $1 billion
2011-01-11
New Rochelle, NY, January 10, 2011—The mega-mergers of 2009 did not continue into 2010. While the three biggest acquisitions in 2009 each had a price tag of more than $40 billion, only last year's top purchase got above that mark, according to an evaluation of reported deals conducted by Genetic Engineering & Biotechnology News (GEN) (http://www.genengnews.com/). The only other mega takeover for the year, sanofi-aventis' move to buy Genzyme, is still being worked out.
A look at 2010's buyouts that crossed the $1billion mark (http://www.genengnews.com/gen-news-highlights/acquisition-deals-in-2010-that-topped-1b/81244443/) ...
Abstinence, heavy drinking, binge drinking associated with increased risk of cognitive impairment
2011-01-11
Amsterdam, The Netherlands, January 10, 2011 -- Previous research regarding the association between alcohol consumption and dementia or cognitive impairment in later life suggests that mild to moderate alcohol consumption might be protective of dementia. However, most of the research has been conducted on subjects already rather elderly at the start of the follow-up. A new study published in the December issue of the Journal of Alzheimer's Disease addresses this problem with a follow-up of more than two decades.
The study, conducted at the University of Turku, University ...
Nuclear receptors reveal possible interventions for cancer, obesity
2011-01-11
HOUSTON, Jan. 10, 2011 – Research with significant implications in the treatment and intervention of cancer and obesity has been published recently in two prestigious journals by University of Houston (UH) biochemist Dr. Jan-Åke Gustafsson.
In an invited review in the Journal of Biological Chemistry, the most-cited biomedical research journal in the world, Gustafsson and his team summarize the most recent results pertaining to the function of a nuclear receptor called estrogen receptor beta, or ERbeta, the biological and medical importance of which Gustafsson and his ...
CT helps identify bullet trajectories
2011-01-11
OAK BROOK, Ill. – Multidetector computed tomography (MDCT) provides an efficient, effective way to analyze wounds from bullets and explosive devices, according to a study published online and in the March issue of Radiology.
"The information provided by MDCT has the potential to improve patient care and aid in both military and civilian forensic investigations," said the study's lead author, Les R. Folio, D.O., M.P.H., from the Uniformed Services University in Bethesda, Md.
U.S. troops stationed in Iraq and Afghanistan face threats from increased sniper activity and ...
Shellfish safer to eat thanks to breakthrough by Queen's scientists
2011-01-11
New technology to make shellfish safer to eat has been pioneered by scientists at Queen's University Belfast.
The new test, developed at Queen's Institute for Agri-Food and Land Use, not only ensures shellfish are free of toxins before they reach the food chain but is likely to revolutionise the global fishing industry.
While the current process for monitoring potentially dangerous toxins in shellfish takes up to two days, the new test slashes the testing time to just 30 minutes using new biosensor technology and provides a much more reliable result.
The test detects ...
A Resolution You Can Keep -- Bay Area Hypnotherapy of San Francisco Challenges You to Succeed in 2011
2011-01-11
Jim and Lynn Swearingen, Co-Founders of Bay Area Hypnotherapy in San Francisco, want to give you a resolution you can keep! "During the months of January and February", said Lynn, "we are offering a $65 coupon for your first Hypnotherapy session, good toward any goal you wish to achieve. This is a whopping $30 off our usual price for an individual session. We're passing this savings on to you because we want everyone to discover what a powerful part their subconscious plays in directing their lives. We can teach you how to use self-hypnosis to achieve lasting change and, ...
Century 21 All Islands Hawaii Real Estate Agent, Oleg Potemkin, Attends Special Training, Builds Expertise in Luxury Real Estate Market.
2011-01-11
Hawaii real estate agent, Oleg Potemkin with CENTURY 21 All Islands, recently completed a luxury home marketing training course offered by the Institute for Luxury Home Marketing.
The course - which covered such topics as demographics of the affluent, lifestyle segmentation, trends and amenities in today's luxury home product, and creating a marketing plan for the multimillion dollar property - was taught by Laurie Moore-Moore, President of the Dallas-based Institute for Luxury Home Marketing and author of the book, Rich Buyer, Rich Seller! The Real Estate Agents' Guide ...
LAST 30 PRESS RELEASES:
New research delves into the potential for AI to improve radiology workflows and healthcare delivery
Rice selected to lead US Space Force Strategic Technology Institute 4
A new clue to how the body detects physical force
Climate projections warn 20% of Colombia’s cocoa-growing areas could be lost by 2050, but adaptation options remain
New poll: American Heart Association most trusted public health source after personal physician
New ethanol-assisted catalyst design dramatically improves low-temperature nitrogen oxide removal
New review highlights overlooked role of soil erosion in the global nitrogen cycle
Biochar type shapes how water moves through phosphorus rich vegetable soils
Why does the body deem some foods safe and others unsafe?
Report examines cancer care access for Native patients
New book examines how COVID-19 crisis entrenched inequality for women around the world
Evolved robots are born to run and refuse to die
Study finds shared genetic roots of MS across diverse ancestries
Endocrine Society elects Wu as 2027-2028 President
Broad pay ranges in job postings linked to fewer female applicants
How to make magnets act like graphene
The hidden cost of ‘bullshit’ corporate speak
Greaux Healthy Day declared in Lake Charles: Pennington Biomedical’s Greaux Healthy Initiative highlights childhood obesity challenge in SWLA
Into the heart of a dynamical neutron star
The weight of stress: Helping parents may protect children from obesity
Cost of physical therapy varies widely from state-to-state
Material previously thought to be quantum is actually new, nonquantum state of matter
Employment of people with disabilities declines in february
Peter WT Pisters, MD, honored with Charles M. Balch, MD, Distinguished Service Award from Society of Surgical Oncology
Rare pancreatic tumor case suggests distinctive calcification patterns in solid pseudopapillary neoplasms
Tubulin prevents toxic protein clumps in the brain, fighting back neurodegeneration
Less trippy, more therapeutic ‘magic mushrooms’
Concrete as a carbon sink
RESPIN launches new online course to bridge the gap between science and global environmental policy
Electric field tunes vibrations to ease heat transfer
[Press-News.org] New method takes snapshots of proteins as they foldScientists have invented a way to 'watch' proteins fold -- in less than thousandths of a second -- into the elaborate twisted shapes that determine their function