(Press-News.org) ABSTRACT CT013
SAN DIEGO ― Combining the KRAS G12C inhibitor adagrasib with the anti-EGFR antibody cetuximab demonstrated promising anti-tumor effects in patients with KRAS G12C-mutated metastatic colorectal cancer (CRC), according to pooled results from the Phase I/II KRYSTAL-1 trial reported by researchers from The University of Texas MD Anderson Cancer Center.
The findings were presented today in a plenary session at the American Association for Cancer Research (AACR) Annual Meeting 2024 by Scott Kopetz, M.D., Ph.D., professor of Gastrointestinal Medical Oncology and associate vice president of Translational Integration, and published in Cancer Discovery, a journal of the AACR.
The targeted therapy combination achieved a disease control rate of 85.1%, an overall response rate of 34%, and a median duration of tumor response of 5.8 months. Median progression-free survival (PFS) was 6.9 months and median overall survival (OS) was 15.9 months.
Based on preliminary results from the Phase I trial, the Food and Drug Administration (FDA) granted Breakthrough Therapy designation to this combination.
“This population of patients with KRAS-mutated colorectal cancer faces a poor prognosis, and there currently are no effective targeted therapies,” Kopetz said. “Therefore, it’s promising to see that this combination shows clinically meaningful benefit both in response rates and duration of disease control along with a very encouraging safety profile.”
KRYSTAL-1 is a multi-center Phase 1/2 trial testing the safety and efficacy of adagrasib alone or in combination with other anticancer therapies in patients with KRAS G12C-mutated metastatic solid tumors. In these two cohorts presented at AACR, the combination of adagrasib plus cetuximab showed antitumor activity and was well tolerated in previously treated patients with KRAS G12C-mutated metastatic CRC, meeting its primary endpoints of safety and objective response rate.
KRAS G12C mutations occur in approximately 3-4% of patients with CRC and are associated with shorter PFS and OS when treated with standard of care chemotherapy, highlighting a critical unmet need.
Adagrasib is a selective and irreversible KRAS G12C inhibitor which can penetrate through the central nervous system, and it previously was shown to be effective for patients with KRAS G12C-mutated non-small cell lung cancer (NSCLC) and untreated brain metastasis as part of the same trial. The FDA granted accelerated approval to adagrasib for the treatment of certain patients with advanced KRAS G12C-mutant NSCLC, with continued approval contingent on verifying clinical benefit in a confirmatory trial.
Adagrasib monotherapy has shown limited activity in patients with KRAS G12C-mutated CRC, and some CRC patients develop resistance to KRAS G12C inhibitor treatment via epidermal growth factor receptor (EGFR)-mediated reactivation of the MAPK signaling pathway. Preclinical data suggested that dual blockade of KRAS G12C with an EGFR inhibitor could help overcome that resistance, leading to evaluation of adagrasib and cetuximab.
The study included 94 heavily pretreated patients with unresectable or metastatic KRAS G12C-mutated CRC with limited treatment options. Participants had a median age of 57 years and 53% were female.
All patients experienced side effects consistent with previous reports on the safety profile of both drugs, with 72.3% of patients experiencing low-grade and 27.7% of patients experiencing more moderate adverse events. Dose reductions of adagrasib and cetuximab occurred in 28 (29.8%) and 6 (6.4%) of patients, respectively. Eight patients (8.5%) discontinued the use of cetuximab due to side effects, but none led to adagrasib discontinuation.
Further exploratory analysis showed that baseline KRAS G12C mutations were detected in the circulating tumor DNA (ctDNA) of 69 out of 83 patients, with 83% concordance between paired tumor samples. This highlights the potential future use of liquid biopsies as a means of stratifying patients most likely to respond well to the combination regimen.
“These results suggest this combination treatment should be considered as a late-line treatment for patients with unresectable or metastatic KRAS G12C-mutated CRC,” Kopetz said.
Kopetz noted that the combination now is recommended by the National Comprehensive Cancer Network (NCCN) Guidelines, and that Phase III trials are currently ongoing.
The study was sponsored by Mirati Therapeutics, Inc. a Bristol Myers Squibb company, and Kopetz reports providing consulting to Mirati. A full list of collaborating authors and their disclosures can be found with the full paper here.
Read this press release in the MD Anderson Newsroom. Information on all MD Anderson AACR Annual Meeting content can be found at MDAnderson.org/AACR.
- 30 -
END
ABSTRACT CT014
SAN DIEGO – The first-in-class PARP1-selective inhibitor saruparib demonstrated encouraging early efficacy and a favorable safety profile in patients with homologous recombination repair (HRR)-deficient breast cancers, according to results from the Phase I/II PETRA trial led by researchers at The University of Texas MD Anderson Cancer Center.
Results from the first-in-human trial were presented today at the American Association for Cancer Research (AACR) Annual Meeting 2024 by Timothy Yap, M.B.B.S., Ph.D., professor ...
LOS ANGELES — Researchers with City of Hope®, one of the largest cancer research and treatment organizations in the United States, will present more than 70 abstracts and sessions on innovative clinical trial results, breakthrough diagnostic techniques and advances in treatment options as well as share their expertise on molecular profiling and the microbiome at the AACR Annual Meeting, which started April 5 and ends April 10 in San Diego.
In addition to City of Hope’s robust data being presented throughout the meeting, John D. Carpten, Ph.D., the Irell & Manella Cancer Center Director’s ...
SAN DIEGO – A combination of the KRASG12C inhibitor adagrasib (Krazati) and the anti-epidermal growth factor receptor (EGFR) antibody cetuximab (Erbitux) showed clinical activity and promising survival outcomes in a cohort of patients with metastatic, heavily pretreated, KRASG12C-mutated colorectal cancer, according to results from the phase I/II KRYSTAL-1 trial presented at the American Association for Cancer Research (AACR) Annual Meeting 2024, held April 5-10.
The study was simultaneously published in Cancer Discovery.
KRASG12C mutations occur in around 4% of colorectal cancers and are associated with a poor prognosis. Drugs targeting KRASG12C, such as adagrasib, have emerged ...
SAN DIEGO – Saruparib, a selective inhibitor of poly-ADP ribose polymerase 1 (PARP1), demonstrated a promising objective response rate and progression-free survival in patients with certain homologous recombination repair (HRR)-deficient breast cancers, according to results from the phase I/II PETRA trial presented at the American Association for Cancer Research (AACR) Annual Meeting 2024, held April 5-10.
Although blocking the enzyme PARP1 may be sufficient to prevent DNA repair in HRR-deficient tumors, all PARP inhibitors currently approved by the ...
SAN DIEGO – An investigational exosome-based liquid biopsy accurately detected 97% of stage 1-2 pancreatic cancers when combined with the biomarker CA 19-9, according to research presented at the American Association for Cancer Research (AACR) Annual Meeting 2024, held April 5-10.
“Pancreatic cancer is one of the most fatal malignancies, in large part because the majority of patients are diagnosed only after the cancer has already metastasized,” said Ajay Goel, PhD, senior author of the study and the chair of the Department of Molecular ...
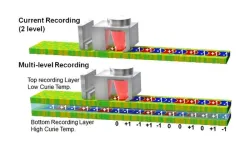
1. Research groups from NIMS, Seagate Technology, and Tohoku University have made a breakthrough in the field of hard disk drives (HDD) by demonstrating the feasibility of multi-level recording using a three-dimensional magnetic recording medium to store digital information. The research groups have shown that this technology can be used to increase the storage capacity of HDDs, which could lead to more efficient and cost-effective data storage solutions in the future.
2. Data centers are increasingly storing vast amounts of data on hard disk drives (HDDs) that use perpendicular ...
While 3D printing has exploded in popularity, many of the plastic materials these printers use to create objects cannot be easily recycled. While new sustainable materials are emerging for use in 3D printing, they remain difficult to adopt because 3D printer settings need to be adjusted for each material, a process generally done by hand.
To print a new material from scratch, one must typically set up to 100 parameters in software that controls how the printer will extrude the material as it fabricates an object. Commonly ...
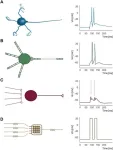
The biological brain, especially the human brain, is a desirable computing system that consumes little energy and runs at high efficiency. To build a computing system just as good, many neuromorphic scientists focus on designing hardware components intended to mimic the elusive learning mechanism of the brain. Recently, a research team has approached the goal from a different angle, focusing on measuring information transfer instead. Their method went through biological and simulation experiments and then proved effective in an electronic neuromorphic system. It was published ...
Philadelphia, April 8, 2024 – Researchers from Children’s Hospital of Philadelphia (CHOP) found that teens and young adults with mood disorders, such as depression and bipolar disorder, were 30% less likely to obtain their driver’s license than peers without such disorders. Additionally, those youths with mood disorders experienced a slightly elevated risk of crashing. These findings suggest that these teens and young adults could benefit from guidance on obtaining licensure and accessing training to avoid crashes when they are newly licensed.
The findings were published online today ...
NEW YORK, NY (April 8, 2024)--A new type of investigational therapeutic in development for pancreatic cancer has shown unprecedented tumor-fighting abilities in preclinical models of the disease, suggesting it has the potential to offer novel treatment options for nearly all pancreatic tumors, a comprehensive study has found.
The inhibitors in this new class of oral medications, being developed by Revolution Medicines Inc., target the oncogenic or active cancer-causing form of RAS proteins (such as KRAS, NRAS, and HRAS). These RAS “oncoproteins” drive up to a third of all human cancers. The research ...