(Press-News.org) Inside cells, it's like in a packed dance club: hundreds are partying. Some keep to themselves, others make their way through the crowd, chatting to everyone they meet. Some just say a quick hello, others stay with their best friends. In this club, there are all kinds of different interactions between party-goers. The same is the case in cells with proteins.
Cells are filled with many different types of proteins that interact with each other and often work together in groups. These groups are called complexes and are molecular machines that only function properly when their individual components interact.
Party-crasher interrupts normal interaction
Which proteins interact with each other and how also depends on the state of the body. Under normal conditions in a healthy body, two proteins, which we call blue and red, join together. If the conditions change due to cellular stress, for example, protein blue can change its interaction partner and join forces with protein yellow, which causes nothing but trouble and disrupts the party.
“Altered interactions between proteins can lead to diseases such as Alzheimer’s, Parkinson’s or cancer,” explains Cathy Marulli. She is a doctoral student under Paola Picotti, a professor at the Institute for Molecular Systems Biology at ETH Zurich. “It is therefore important to know how protein-protein interactions differ between healthy and diseased states and what the binding sites between two proteins look like. If we know these down to the last detail, we can develop active substances that block unwanted interactions and restore the cell’s equilibrium,” she explains.
Revealing the social network of proteins
The ETH biochemists have therefore further developed a proven approach in protein research to analyse the complete interaction network of proteins, known as the interactome.
The corresponding study has just been published in the journal Nature Biotechnology.
Several years ago, Picotti and her colleagues developed what is known as LiP mass spectrometry. This enables researchers to measure structural changes in thousands of proteins in any biological sample, without the samples having to be specially purified beforehand. They last used this method to analyse proteins and their functions (see ETH News).
Now they have further developed LiP mass spectrometry to study the interactions between proteins. To this end, they first identified around 6,000 interaction interfaces between proteins and other sites that change when proteins interact with each other. They then used these sites as markers to assess whether a protein changes its interaction with other proteins under a certain condition.
To do this, they used enzymes that cut proteins into pieces. These enzymes can only attack proteins at freely accessible sites. The enzyme cannot cut a protein if another protein is docked at a site. Detailed information on the protein fragments therefore helped the researchers to analyse whether and where individual proteins interact with others. This enabled them to study the interactions of about 1,000 proteins simultaneously and directly in a messy cell matrix.
Striking changes in stressed cells
The researchers worked with yeast cells to study how the interactions of proteins differ in their normal state from those in a stress situation triggered by a chemical substance.
In so doing, the biochemists discovered that the stress situation had altered around five dozen protein complexes and thus their interactions. The researchers also showed that a protein complex called SAGA plays an important role in the interaction network of the yeast cell. When they removed SAGA from the picture, around two-thirds of the protein complexes behaved differently in the stress situation. “SAGA is the DJ at the party. When it is muted, some party groups stop dancing. They influence other party-goers, who also withdraw. This shows that a single player in the cell has a disproportionately large influence on others,” says Marulli.
Transferable to other species
The method developed can also be applied to other organisms. “For each species we want to study, we just need to develop a new set of binding markers to be able to use this method to study protein interactions in mouse or human cells,” says Marulli. The next logical step is therefore to determine the interaction markers for the interactome of human cells in order to analyse defective protein interactions in a single step.
Determining protein interactions is extremely important in relation to diseases. “We therefore want to further develop our technology for diagnostic purposes and for research into disease mechanisms,” says Picotti. There is a good reason for this hope: previous approaches developed in their laboratory have already been put into practice by ETH spin-off Biognosys.
Pharmaceutical research targets interactions
Pharmaceutical research is also very interested in the interaction markers. If the interaction sites are known, researchers can efficiently search for chemical compounds that can interrupt unwanted interactions or establish new ones.
Compounds that modulate protein-protein interactions are currently a promising new direction in pharmaceutical research. Such compounds could potentially address proteins that are not accessible with current drugs. Or they can be used to develop new drugs with fewer side effects.
END
Protein interactions: Who is partying with whom and who is ruining the party?
2024-10-16
ELSE PRESS RELEASES FROM THIS DATE:
New biochar nanocomposite enhances detection of acetaminophen and uric acid in urine
2024-10-16
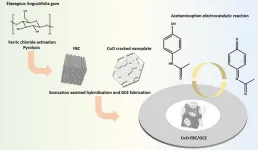
In recent years, the excessive use of acetaminophen (APAP) has become a significant human hazard and social burden. Rapid and automated electrochemical detection has emerged as a crucial method for measuring APAP concentration in human urine. This study explores a novel porous cobalt-derived biomass electrocatalyst material prepared from Elaeagnus angustifolia gum and investigates its electrochemical properties as well as its specific detection capability for APAP. Their work is published in the journal Industrial Chemistry & Materials on 18 July 2024.
Acetaminophen (APAP) is a commonly used analgesic and antipyretic ...
F. William Studier receives the 2024 Merkin Prize in ceremony at the Broad Institute for developing technology used to produce millions of doses of COVID-19 vaccines
2024-10-16
The 2024 Richard N. Merkin Prize in Biomedical Technology was awarded to F. William Studier of Brookhaven National Laboratory in a ceremony and symposium at the Broad Institute on September 17, 2024. The prize, created by the Merkin Family Foundation and administered by the Broad, recognizes novel technologies that have significantly improved human health and carries a $400,000 award. [Event Video]
Studier was announced as the winner in May for his development, in 1986, of an efficient, scalable method of producing ...
Applications open for School of Advanced Science on Structural Safety
2024-10-16
The “São Paulo School of Advanced Science on Structural Safety and its role in reducing greenhouse gas emissions by the built environment” will be held May 3-16, 2025, in Brazil, at the São Carlos School of Engineering of the University of São Paulo in its São Carlos campus.
Reporters are invited to register for the scientific sessions and short courses, which will present state-of-art science and results of new research.
This advanced science school will address the main challenges related to Structural Safety ...
Scientists use Allen Telescope Array to search for radio signals in the TRAPPIST-1 star system
2024-10-16
October 16, 2024, Mountain View, CA - Scientists at the SETI Institute and partners from Penn State University used the Allen Telescope Array (ATA) to search for signs of alien technology in the TRAPPIST-1 star system. The team spent 28 hours scanning the system, looking for radio signals that could indicate extraterrestrial technology. This project marks the longest single-target search for radio signals from TRAPPIST-1. Although they didn’t find any evidence of extraterrestrial technology, their work provided valuable data ...
Zebrafish as a model for studying rare genetic disease
2024-10-16
Fukuoka, Japan—Nager syndrome, or NS, is a rare genetic disease that affects the development of the face and limbs, usually causing anomalies in the bone structures of the jaws, cheeks, and hands. With a prevalence of less than 100 cases ever reported, not much is known about the disease except the fact that mutations in the SF3B4 gene are its primary cause. Now, in a recent study made available online on September 15, 2024 and upcoming in the November issue of International Journal of Biological Macromolecules, researchers from Kyushu University have developed a convenient approach to explore the underlying mechanisms ...
A synthetic molecular switch lets you 'paint' with natural light
2024-10-16
Liquid crystals exist in a phase of their own. They can flow like liquids, but because their molecules are arranged in a somewhat orderly way, they can be easily manipulated to reflect light. This flexibility has made liquid crystals the go-to material for energy-efficient phone, TV, and computer display screens.
In a new study in Nature Chemistry, researchers at Dartmouth and Southern Methodist University hint at other applications for liquid crystals that might one day be possible, all powered by natural light. They include liquid crystal ...
Honoring a career of outstanding achievement
2024-10-16
NEWPORT NEWS, VA – This year, the U.S. Department of Energy's Thomas Jefferson National Accelerator Facility celebrates the 40th anniversary of its founding to probe the secrets of the subatomic universe. And for 39 of those years, esteemed physicist Volker D. Burkert has been an important part of its mission.
Now, Burkert is being honored for his contributions to advancements in experimental physics with the prestigious Tom W. Bonner Prize in Nuclear Physics. The citation reads: “For exemplary leadership in the development of high-performance instrumentation for large acceptance spectrometers that have enabled breakthroughs in fundamental nuclear ...
MSK research highlights, October 15, 2024
2024-10-16
A new tactic for overcoming breast cancer drug resistance
The most common type of breast cancer, estrogen receptor positive, has been effectively treated with hormone therapy combined with drugs that block cell division called CDK4/6 inhibitors. However, it has been impossible to predict how long people will respond to this drug combination. In some patients, the disease is controlled for years, but in others, the cancer starts progressing again after just a few months. This presents ...
Hot news flash: Menopause can impact a woman’s heart health
2024-10-16
DALLAS, Oct. 16, 2024 — The risk of heart disease increases with age for most people, however, for women that may be even more true. The menopause transition, those years leading up to and through menopause, is a time of increasing heart disease risk, according to an American Heart Association scientific statement published in the flagship journal Circulation in 2020.
“While many people think that breast cancer is the leading killer of women in the U.S., in ...
Standing more may not reduce cardiovascular disease risk, could increase circulatory disease
2024-10-16
EMBARGO: SYDNEY: 17 October 2024, 00.01 | NEW YORK: 16 October 2024, 09:00
Standing more may not reduce cardiovascular disease risk, could increase circulatory disease
Standing has gained popularity among people looking to offset the harms of a sedentary lifestyle often caused by spending long days sitting in front of the computer, television or driving wheel. Standing desks have become a popular option among office workers, and in other industries like retail, workers may opt to stand instead of sit.
However, their efforts may not produce the intended result. New University of Sydney research has shown that over ...