(Press-News.org) WHAT:
A clinical trial sponsored by the National Institutes of Health (NIH) has launched to examine the safety and acceptability of a novel rectal HIV microbicide douche containing the antiretroviral drug tenofovir. This “on-demand” HIV prevention approach involves using the microbicide prior to a potential exposure from receptive anal intercourse.
Several forms of HIV pre-exposure prophylaxis (PrEP) are in use in the United States and globally, namely daily oral pills, long-acting injections, and a monthly vaginal ring. The Centers for Disease Control and Prevention advises that gay, bisexual and other men who have sex with men who meet certain criteria can take “on-demand” oral PrEP around the time of sex to prevent HIV acquisition, but there is insufficient evidence to support its use in other populations. Rectal microbicides are another HIV prevention method being explored for use in an “on-demand” manner to expand the choices available to eligible people who engage in receptive anal intercourse and who stand to benefit from using PrEP.
The clinical trial will enroll about 150 adults assigned male at birth who have regular experience using an unmedicated rectal douche before receptive anal intercourse. Participants will each receive “on-demand” tenofovir rectal microbicide douche during one two-month period and on-demand oral PrEP with tenofovir disoproxil fumarate and emtricitabine in another two-month period. All particpants will be monitored closely for safety. The study also will assess participants’ experience using their assigned PrEP method, including measures of acceptability, adherence, and method preference. The study will take place at eight sites in the United States.
While HIV incidence is slowly decreasing in the United States, 67% of U.S. HIV diagnoses from 2018-2022 were among gay, bisexual, and other men who have sex with men, pointing to the need for expanded HIV prevention options. The mid-stage study is sponsored NIH’s National Institute of Allergy and Infectious Diseases (NIAID) and implemented through the NIH-funded HIV Prevention Trials Network (HPTN). NIH remains committed to developing safe and effective HIV prevention methods that people need, want, and are able to use.
More information about this study, also known as HPTN 106, is available at ClinicalTrials.gov under identifier NCT06560684.
WHO:
Sheryl Zwerski, D.N.P., director of the Prevention Sciences Program in NIAID’s Division of AIDS, is available to discuss this research.
CONTACT:
To schedule interviews, please contact NIAID News & Science Writing Branch, 301-402-1663, niaidnews@niaid.nih.gov.
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit https://www.nih.gov/.
NIH...Turning Discovery Into Health®
END
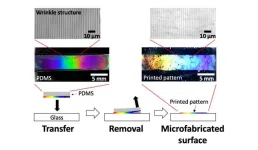
1. A team of researchers from NIMS and the University of Connecticut has developed a printing technique capable of forming a periodic nano/microstructure on the surface of a polydimethylsiloxane (PDMS) slab and easily transferring it onto the surface of a glass substrate. This technique enables us to create materials with useful functions—including water-repellency and the ability to generate structural colors—without expensive equipment and complex processes. In addition, the technique may be used to fabricate materials capable of realizing anti-fogging and/or generating structural colors on their surfaces—functions potentially useful in the development ...
Reports of drug-related supply-chain issues were 40% less likely to result in drug shortages in Canada versus the United States, according to a new study from University of Pittsburgh researchers and published today in JAMA.
The analysis looked at drugs that had reports of supply-chain disruptions between 2017 and 2021 in both countries and found that within 12 months of an initial U.S. report, nearly half resulted in drug shortages in the U.S. versus about one-third in Canada. There was also a consistently lower ...
About The Study: Drug-related reports of supply chain issues were 40% less likely to result in meaningful drug shortages in Canada compared with the U.S. These findings highlight the need for international cooperation between countries to curb the effects of drug shortages and improve resiliency of the supply chain for drugs.
Quote from corresponding author Katie J. Suda, PharmD, MS:
“Our U.S. drug supply chain is linked globally – shortages in one country can happen in another country – presenting an opportunity to compare and ...
About The Study: The results of this prospective cohort study suggest that evidence-based smoking cessation treatment within 6 months following a cancer diagnosis maximizes survival benefit. This study supports smoking cessation as an important early clinical intervention for patients after being diagnosed with cancer.
Corresponding Author: To contact the corresponding author, Paul M. Cinciripini, PhD, email pcinciri@mdanderson.org.
To access the embargoed study: Visit our For The Media website at this link ...
HOUSTON ― Smokers who are diagnosed with cancer now have more incentive to quit, as researchers from The University of Texas MD Anderson Cancer Center have found survival outcomes were optimized when patients quit smoking within six months of their diagnosis.
Study results, published today in JAMA Oncology, found a 22%-26% reduction in cancer-related mortality among those who had quit smoking within three months after tobacco treatment began. The best outcomes were observed in patients who started tobacco treatment within six months of a cancer diagnosis and were abstinent from smoking three months later. Survival for these patients increased from 2.1 years for ...
CONTACT: Heide Aungst
HAungst@som.umaryland.edu
(216) 970-5773 (cell)
UNDER EMBARGO UNTIL 11 am on OCT. 31
Genomic Databases Need More Diversity
University of Maryland School of Medicine Researchers Create Large Database of Latin American Populations to Tackle Health Disparities
BALTIMORE, Oct. 31, 2024: It is commonly known that most genomic databases are biased toward people with European ancestry. Scientists have warned that leaving out other populations could skew results in areas such as drug development, ...
Recent rules that require all new building and road projects in England to address and offset their impact on nature are excellent in principle but flawed in their implementation, leading environmental economists argue.
Under Biodiversity Net Gain (BNG), which became law this year, new building or infrastructure developments must achieve a 10% net gain in biodiversity or habitat.
In a new study published in One Earth, experts criticise the implementation of the policy which forces the majority of off-setting to occur within or near development sites rather than where it might most ...
INDIANAPOLIS – Although much rarer than either breast or prostate cancers, testicular cancer is the most common solid tumor in males between the ages of 15 and 35, with approximately 10,000 young men diagnosed annually in the United States.
With the goals of informing surgical management, improving long-term outcomes and lowering death rates of patients with testicular cancer, a study led by urologist and health services researcher Clint Cary, M.D., MPH, MBA, of the Indiana University School of Medicine and the Regenstrief ...
Dr. Chani Traube, the Gerald M. Loughlin, MD Professor of Pediatrics at Weill Cornell Medicine, has been awarded a $3.4 million grant, with the possibility of extending to a total of $17 million over five years, from the National Institutes of Health, for a large-scale clinical trial called Optimizing Pain Treatment in Children on Mechanical ventilation (OPTICOM).
OPTICOM, funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, is part of the NIH’s HEAL KIDS PAIN initiative. The OPTICOM study will enroll 644 children in 14 pediatric intensive care units across the United States that are part of the institute’s ...
The Chan Zuckerberg Biohub San Francisco (CZ Biohub SF) and The 15 White Coats, Inc., have launched two initiatives that will provide travel grants as well as coursework in metagenomic sequencing and genomic epidemiology to aspiring physicians from underrepresented groups. CZ Biohub SF is one of a group of research institutes created and supported by the Chan Zuckerberg Initiative (CZI).
The new initiatives are driven by CZ Biohub SF’s Rapid Response Team, which offers training, tools, and technologies to help build sustainable scientific relationships—with a special emphasis on the use of genomic sequencing platforms for pathogen discovery and detection—in laboratories ...