This roundup highlights the biology behind our sense of smell, real-world data that can be used to refine esophageal cancer treatment guidelines, a potential new approach to treating patients with type 2 diabetes, a new way to target pancreatic cancer and the start of what could become “off-the-shelf” immunotherapies for cancer.
To learn more about research at City of Hope, one of the largest and most advanced cancer research and treatment organizations in the United States and ranked among the nation’s top 5 cancer centers by U.S. News & World Report, visit the Research & Innovation page.
How our nose “knows”: Unraveling the biology behind our sense of smell
The human nose teems with some 400 different receptors that help us sniff out smells at the molecular level. Scientists created 3D models of four of these receptors to learn how they detect different scents, according to a Nature study.
Co-led by Nagarajan Vaidehi, Ph.D., City of Hope chair of computational and quantitative medicine, the researchers used advanced imaging techniques to watch the engineered receptors interact with various smells and applied computational methods to understand how the receptors recognize odors. The team pinpointed two main types of receptors: one that primarily reacts to sour-smelling substances and a larger group that can identify a wide variety of odors.
The City of Hope team showed how the engineered 3D receptors closely resembled their human counterpart, suggesting they could help scientists understand how human odorant receptors work. Overall, the findings increase scientific understanding of how our nose perceives scents and could lead to new insights into the biology behind our sense of smell. Equally important, the engineered receptors provide a valuable tool for future olfactory research.
For more information, read the Nature study.
Study of real-world outcomes confirms a new way of treating esophageal cancer
Current clinical trials consider trimodality therapy, or chemotherapy and radiation followed by surgery, as the gold standard for treating esophageal adenocarcinoma cancer (EAC). Recently new data has emerged suggesting perioperative chemotherapy — chemotherapy before and after surgery — may be a more effective treatment. Now a new JAMA study suggests that perioperative chemotherapy before surgery may be effective at enhancing patient survival in the real-world setting.
Led by City of Hope’s Dani Castillo, M.D., City of Hope assistant clinical professor of medical oncology and therapeutics research, and S. Peter Wu, M.D., City of Hope assistant clinical professor of radiation oncology, the researchers explored survival rates among EAC patients across the United States. Using retrospective data from the National Cancer Database, the team focused on 57,116 patients treated for Stage II or III EAC from January 2006 to December 2020. The results were eye-opening.
On average patients who underwent
Chemotherapy before and after surgery resulted in median overall survival of 66.2 months. Trimodality therapy resulted in median overall survival by 43.9 months. Chemoradiation alone resulted in a median overall survival rate of 18.1 months. Radiation resulted in median overall survival of only 13.6 months. This study represents the first real-world data in the U.S. that is aligned with recent randomized trials in this field, adding a unique perspective to the existing literature. By providing insights grounded in a U.S. cohort, the findings contribute to a broader understanding of the value of real-world outcomes, potentially helping to refine practice guidelines to the benefit of patients.
For more information, read the JAMA study.
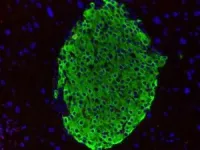
Boosting PAK1 in beta cells mitigates diet-induced high blood sugar in mice
Beta cells in our pancreas make insulin, a hormone crucial for regulating blood sugar. A protein called PAK1 keeps beta cells healthy and plentiful, but PAK1 levels drop 80% in people with type 2 diabetes. A City of Hope team hypothesized that increasing PAK1 could sustain the supply of beta cells, help them produce more insulin and reverse high blood sugar levels.
Led by Debbie Thurmond, Ph.D., the Chan Soon-Shiong Shapiro Distinguished Chair in Diabetes and director of the Arthur Riggs Diabetes & Metabolism Research Institute at City of Hope, along with Miwon Ahn, Ph.D., City of Hope assistant research professor, researchers fed genetically modified mice a high-fat diet, restricting the ability to maintain healthy control of their blood sugar. After three weeks, the scientists added doxycycline to the food of the animals with the highest blood sugar levels for another three weeks. The antibiotic switched on the PAK1 gene only in the mice’s beta cells, triggering them to produce the PAK1 protein. A second group of mice continued the high-fat diet without doxycycline, while a third group ate regular chow with doxycycline.
At the end of the experiment, scientists collected samples from each animal’s pancreas to analyze the beta cells’ ability to release insulin when exposed to glucose. The mice who received PAK1 had better blood sugar control and had the capacity to make more insulin than the animals who did not receive PAK1. Boosting PAK1 also prevented beta cell death and enhanced the function of genes that support insulin production. By confirming that PAK1 plays a vital role in regulating blood sugar, the findings suggest a fundamental new approach to treating type 2 diabetes.
For more information, see the Diabetologia study.
Targeting a novel vulnerability to disarm a stubborn form of pancreatic cancer
With a five-year mortality rate of 90%, pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest human cancers. Now City of Hope scientists have uncovered a potential vulnerability in PDAC that could be exploited for therapy, according to a Cell Reports study.
When cancer-driving genes are mutated in PDAC, the cells are driven to read and copy genes at a much higher rate, causing the gene reading and copying machineries to collide. This process is called transcription replication conflicts (TRCs). How pancreatic cancer cells can withstand these collisions without dying was not known.
Led by Mustafa Raoof, M.D., M.S., City of Hope assistant professor of surgery, cancer genetics and epigenetics, researchers sought to identify mechanisms that allow PDAC cells to manage a high level of TRCs. They discovered that a cellular repair process called the base-excision repair (BER) pathway plays a crucial role in managing TRCs. Blocking the BER pathway worsened TRCs, increasing DNA damage that triggered a cellular stress response. When the team used drugs to simultaneously block the BER pathway and the stress response, the resulting DNA damage killed the cancer cells. By unraveling how PDAC cells react to TRCs and discovering a way to defuse the adaptive mechanism, the findings offer potential new avenues for treatment.
For more information, read the Cell Reports study.
Checkpoint knockout could lead to potent, ready-to-use immunotherapies for cancer
Immunotherapy boosts a person’s own immune system to battle cancer, but cancer cells often find ways to evade the body's attack. One way is by exploiting checkpoints that fool the immune system into not recognizing or targeting cancer cells, thereby protecting the cells from assault. City of Hope scientists focused on a checkpoint called NKG2A on the immune system’s natural killer (NK) cells that fight off cancer and infection.
Led by Yue Qin, Ph.D., Qi Cui, Ph.D., and Yanhong Shi, Ph.D., City of Hope chair of neurodegenerative diseases, director of stem cell biology research and the Herbert Horvitz Professor in Neuroscience, the research team genetically modified human pluripotent stem cells to remove NKG2A and transform them into NK cells, according to a Cell Reports study. The researchers found that the modified NK cells more effectively destroyed glioblastoma and leukemia cells, as well as cells infected with the COVID-19 virus. When tested in laboratory mice with tumors, the NK cells slowed cancer growth and helped the animals live longer.
Easily grown and genetically altered from human stem cells, the modified NK cells suggest a convenient option for future cancer immunotherapies that can be produced in bulk for “off-the-shelf” use when needed. This work was supported by a California Institute for Regenerative Medicine award.
For more information, see the Cell Reports study.
Awards and Honors
Colton Ladbury, M.D., City of Hope assistant professor of radiation oncology, received the inaugural ARRO Resident Educator of the Year Award from the Association of Residents in Radiation Oncology and the American Society for Radiation Oncology.
# # #
About City of Hope
City of Hope's mission is to make hope a reality for all touched by cancer and diabetes. Founded in 1913, City of Hope has grown into one of the largest and most advanced cancer research and treatment organizations in the U.S., and one of the leading research centers for diabetes and other life-threatening illnesses. City of Hope research has been the basis for numerous breakthrough cancer medicines, as well as human synthetic insulin and monoclonal antibodies. With an independent, National Cancer Institute-designated comprehensive cancer center that is ranked top 5 in the nation for cancer care by U.S. News & World Report at its core, City of Hope’s uniquely integrated model spans cancer care, research and development, academics and training, and a broad philanthropy program that powers its work. City of Hope’s growing national system includes its Los Angeles campus, a network of clinical care locations across Southern California, a new cancer center in Orange County, California, and cancer treatment centers and outpatient facilities in the Atlanta, Chicago and Phoenix areas. City of Hope’s affiliated group of organizations includes Translational Genomics Research Institute and AccessHopeTM. For more information about City of Hope, follow us on Facebook, X, YouTube, Instagram and LinkedIn.
END