Gold nanoparticles (AuNPs) are tiny gold particles of 1–100 nanometers and have unique chemical and biological properties. Due to their potential to accumulate in tumors, these nanoparticles have emerged as promising drug carriers for cancer therapy and targeted drug delivery. However, tracking the movement of these nanoparticles in the body has been a major challenge. Traditional imaging methods often involve tracers like fluorescent dyes and radioisotopes, which give limited visualization and inaccurate results due to detachment from AuNPs.
In a step to advance the imaging of AuNPs, researchers from Waseda University introduced a new imaging technique that uses neutron activation to transform stable gold into a radioisotope of gold and enables long-term tracking of the AuNPs within the body. The study was led by Nanase Koshikawa, a PhD student in the Graduate School of Advanced Science and Engineering at Waseda University, and Jun Kataoka, a Professor in the Faculty of Science and Engineering at Waseda University, in collaboration with Osaka University and Kyoto University. The findings of this study were published in Applied Physics Letters on March 12, 2025.
“Traditional imaging methods involve external tracers, which may detach during circulation,” explains Koshikawa. “To overcome this limitation, we directly altered the AuNPs, making them detectable via X-rays and gamma rays without the use of external tracers.”
For activation of the AuNPs, the researchers irradiated the stable gold nanoparticles with neutrons, converting the stable (197Au) to radioactive (198Au). The radioactive 198Au emits gamma rays, which are detectable from outside the body. Prof. Kataoka explains neutron activation, stating, “Activation of atoms through particle irradiation is a technique that directly alters the material. The altered elements are sometimes unstable and emit X-rays and gamma rays that make the material visible from outside the body. This does not change the atomic number, and thus the chemical properties of the element are preserved.”
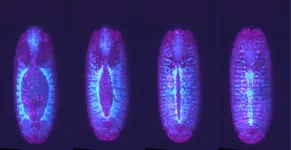
The researchers further confirmed the tracking of these radioactive AuNPs by injecting them into tumor-bearing mice and visualizing them using a special imaging system.
Additionally, the study demonstrated this imaging technique for drug delivery of 211At, a radio-therapeutic drug used in targeted cancer therapy. The 211At emits alpha particles and X-rays, which are detectable for a shorter duration due to a shorter half-life. The researchers labeled the 211At with the radioactive AuNPs, forming 211At-labeled (198Au) AuNPs. This approach provided long-term imaging of the drug due to the longer half-life (2.7 days) of 198Au, overcoming the limitations of the short half-life of 211At.
“211At has a half-life of only 7.2 hours, and hence its emitted X-rays disappear within 2 days, but with the (198Au) AuNPs labeling, we were able to track the drug’s distribution for up to 5 days using gamma rays from ¹⁹⁸Au, which has a longer half-life of 2.7 days,” says co-author Atsushi Toyoshima from the Institute for Radiation Sciences, Osaka University.
This study represents a breakthrough in the field of targeted drug delivery and could lead to major advancements in drug delivery systems. The direct tracking of AuNPs inside the body could pave the way for more effective cancer treatments with precise monitoring of drug distribution. The study could also open new possibilities for real-time pharmacokinetic studies, ensuring improved drug safety and efficacy.
“AuNPs are being actively researched for medical applications,” explains co-author Hiroki Kato from the Institute for Radiation Sciences, Osaka University. “We developed a simple and scalable technique for tracking AuNPs that could significantly advance nanomedicine while driving the optimization of gold-based nanomaterials.”
Reflecting on their plans, co-author Yuichiro Kadonaga, an Assistant Professor from the Institute for Radiation Sciences, Osaka University, shares his perspective, saying, “We plan to enhance the imaging resolution and extend this technique to various nanoparticle-based systems. By further refining neutron activation imaging, we aim to make drug monitoring a clinical reality, potentially revolutionizing the field of imaging technologies.”
***
Reference
Authors: N. Koshikawa,1 Y. Kikuchi,1 K. S. Tanaka,1 K. Tokoi,2 A. Mitsukai,3 H. Aoto,2 Y. Kadonaga,3 A. Toyoshima,3 H. Kato,3 K. Ooe,3 K. Takamiya,4 and J. Kataoka1
Title of original paper: Activation imaging of gold nanoparticles for versatile drug visualization: an in vivo demonstration
Journal: Applied Physics Letters
DOI: 10.1063/5.0251048
Affiliations:
1Graduate School of Advanced Science and Engineering, Waseda University, Japan
2Department of Chemistry, Graduate School of Science, Osaka University, Japan
3Institute for Radiation Sciences, Osaka University, Japan
4Institute for Integrated Radiation and Nuclear Science, Kyoto University, Japan
About Waseda University
Located in the heart of Tokyo, Waseda University is a leading private research university that has long been dedicated to academic excellence, innovative research, and civic engagement at both the local and global levels since 1882. The University has produced many changemakers in its history, including nine prime ministers and many leaders in business, science and technology, literature, sports, and film. Waseda has strong collaborations with overseas research institutions and is committed to advancing cutting-edge research and developing leaders who can contribute to the resolution of complex, global social issues. The University has set a target of achieving a zero-carbon campus by 2032, in line with the Sustainable Development Goals (SDGs) adopted by the United Nations in 2015.
To learn more about Waseda University, visit https://www.waseda.jp/top/en
About Nanase Koshikawa from Waseda University
Ms. Nanase Koshikawa is a PhD student at the Graduate School of Advanced Science and Engineering, Waseda University, Japan. She specializes in advanced imaging techniques for nuclear medicine and is developing high-resolution imaging systems for detecting high-energy gamma rays. In November 2024, she presented a new SPECT system at the 6th International Workshop on New Photon-Detectors (PD24), which overcomes the limitations of traditional SPECT systems, improving accuracy in medical imaging. Her key research areas include high-resolution imaging, photon imaging, and radio imaging.
END