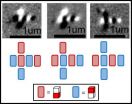
A team of researchers — led by Pierre Gressens, at Inserm U676, Paris, France, and Vincent Lelièvre, at CNRS UPR-3212, Strasbourg, France — has identified a signaling pathway key for normal brain development in the mouse. Of paramount importance, the data generated suggest that environmental factors, including maternal ones, can influence the final size of the brain.
Individuals with microcephaly primary hereditary (MCPH) are born with a very small head and a small brain. They suffer mild developmental delay, hyperkinesia (excessive restlessness), and mild to severe cognitive impairment. Although mutation of any one of seven genes is known to cause MCPH, a lack of good animal models has made it hard to understand the underlying mechanisms. To gain insight into this, Gressens, Lelièvre, and colleagues used a mouse model in which microcephaly is induced by blocking the peptide VIP during gestation using a VIP antagonist (VA). Initial analysis indicated that prenatal administration of VA gives rise to brain abnormalities that mimic those observed in patients with MCPH. Further analysis identified a cellular and molecular mechanism for the observed abnormalities. The authors therefore conclude that the identified molecular pathway (the VIP/Mcph1/Chk1 pathway) is key for normal brain development and suggest that environmental factors disturbing this pathway can modulate the development of the brain as well as its final size.
TITLE: VIP blockade leads to microcephaly in mice via disruption of Mcph1-Chk1 signaling
AUTHOR CONTACT:
Pierre Gressens
Inserm U676, Hôpital Robert Debré, Paris, France.
Phone: 33.1.40.03.19.76; Fax: 33.1.40.03.19.95; E-mail: pierre.gressens@inserm.fr.
Vincent Lelièvre
CNRS UPR-3212, Université de Strasbourg, Strasbourg, France.
Phone: 33.3.88.45.66.59; Fax: 33.3.88.60.16.64; E-mail: lelievre@inci-cnrs.unistra.fr.
View this article at: http://www.jci.org/articles/view/43824?key=1236357151332f9ec680
EDITOR'S PICK: T-ing up a new target for Parkinson disease treatment
Parkinson disease (PD) affects 1-2% of the population over the age of 65 years. It results from loss or loss of function of nerve cells in the brain that coordinate movement. As a result, the hallmark symptoms of PD are trembling in hands, arms, legs, jaw, and face; stiffness of the limbs and trunk; slowness of movement; and impaired balance and coordination. There is no cure for PD, but symptoms can be alleviated with a variety of drugs. A team of researchers, led by Chung-Chin Kuo, at National Taiwan University College of Medicine, Taiwan, has now shown that pharmacological blockade of T-type calcium channels reduces deficiencies in the ability to move normally in a rat model of PD. This, and other observations, leads them to suggest that targeting T-type calcium channels could provide a new approach to treating individuals with PD.
TITLE: Modulation of subthalamic T-type Ca2+ channels remedies locomotor deficits in a rat model of Parkinson disease
AUTHOR CONTACT:
Chung-Chin Kuo
National Taiwan University College of Medicine, Taipei, Taiwan.
Phone: 886.2.23123456, ext. 88236; Fax: 886.2.23964350; E-mail: chungchinkuo@ntu.edu.tw.
View this article at: http://www.jci.org/articles/view/46482?key=b7b5f0630636ab8bf422
ONCOLOGY: Role for stem cells in tumor development
Ovarian cancer is the fifth most common cancer among women. New research, performed by Ronald Buckanovich and colleagues, at the University of Michigan Medical Center, Ann Arbor, provides insight into the cellular and molecular mechanisms that promote ovarian tumor development and thereby identifies a potential new therapeutic approach.
Mesenchymal stem cells (MSCs) are multipotent cells that have the capacity to generate many different cell types, including bone cells, cartilage cells, and fat cells. Strong evidence indicates that MSCs are recruited to tumors, but what they do when they get there is a matter of some controversy. Buckanovich and colleagues now show that carcinoma-associated MSCs (CA-MSCs) are present in the majority of human ovarian tumors and that they themselves are not cancerous, simply in the microenvironment of the tumor. Further analysis indicated that CA-MSCs promote tumor growth by increasing the number of cancer stem cells and that they do this, at least in part, through increased production of growth factors known as BMPs. The authors therefore conclude that MSCs in the ovarian tumor microenvironment contribute to tumor development and suggest that targeting BMPs might provide a new approach to treating ovarian cancer.
TITLE: Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production
AUTHOR CONTACT:
Ronald J. Buckanovich
University of Michigan Medical Center, Ann Arbor, Michigan, USA.
Phone: 734.764.2395; Fax: 734.936.7376; E-mail: ronaldbu@med.umich.edu.
View this article at: http://www.jci.org/articles/view/45273?key=6fd6a37b7c84ac931756
VACCINES: Vitamin A derivative redirects vaccine-activated immune cells to the gut
Diarrheal diseases are a major cause of childhood death in developing countries. They are caused by a wide array of bacteria, parasites, and viruses that infect the gut (these are collectively known as enteropathogens). Injectable vaccines fail to provide protection against enteropathogens because they do not trigger immune cells to travel to the gut. But now, a team of researchers, led by Reinhold Förster, at Hannover Medical School, has developed an approach to direct immune responses activated by a vaccine injected under the skin to the gut. Importantly, this approach (concurrent injection of a vaccine and the molecule retinoic acid [RA] under the skin) protected mice from cholera toxin–induced diarrhea and decreased numbers of bacteria in their gut after oral infection with the bacterium Salmonella. The team therefore suggests that RA or its precursors, which include vitamin A, might be used to trigger immune cells activated by injectable vaccines to navigate to the gut in order to provide protection against enteropathogens.
TITLE: Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice
AUTHOR CONTACT:
Reinhold Förster
Hannover Medical School, Hannover, Germany.
Phone: 49.511.5329733; Fax: 49.511.5329722; E-mail: Foerster.Reinhold@MH-Hannover.de.
View this article at: http://www.jci.org/articles/view/44262?key=26660a014dec788b9889
AUTOIMMUNITY: New recruits slow type 1 diabetes
A team of researchers, led by Bruce Verchere, at the Child and Family Research Institute, Vancouver, has now identified a way to harness a naturally occurring immunosuppressive mechanism to prevent the onset of disease in a mouse model of type 1 diabetes.
Type 1 diabetes occurs when a person's immune system attacks and destroys the cells in the pancreas that produce the hormone insulin. Some researchers are seeking to develop ways to harness the suppressive mechanisms of the patient's own immune system to treat the condition. In this context, cells known as Tregs are of particular interest because they have a pivotal role in suppressing the proliferation and function of other immune cells. Verchere and colleagues used a virus to deliver the genetic information for making the protein CCL22 (a molecule known to attract Tregs) to insulin-producing cells in the pancreas of mice susceptible to a type 1 diabetes–like condition and found that it led to the recruitment of Tregs to the pancreas and long-term protection from diabetes. Importantly, virus-mediated expression of CCL22 in pancreatic cells transplanted into diabetic mice led to recruitment of Tregs to the grafts, prevention of insulin-producing–cell destruction, and delayed diabetes recurrence. The team therefore suggests that delivering Treg attractants to the pancreas may provide a new way to treat type 1 diabetes.
TITLE: Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets
AUTHOR CONTACT:
C. Bruce Verchere
Child and Family Research Institute, Vancouver, British Columbia, Canada.
Phone: 604.875.2490; Fax: 604.875.2373; E-mail: bverchere@cfri.ca.
View this article at: http://www.jci.org/articles/view/43048?key=e02144b90bcf49ac8c33
ALLERGY AND ASTHMA: Stomach-colonizing bacterium protects against asthma
The number of people with asthma has increased sharply over the past few decades. This decrease has been linked to the disappearance of the bacterium Helicobacter pylori, which persistently colonizes the human stomach, from Western societies. A team of researchers — led by Anne Müller, at the University of Zürich, Switzerland and Christian Taube, at Johannes Gutenberg University, Germany — has now provided concrete evidence in mice to support this idea and identified an underlying mechanism to explain the beneficial effect of Helicobacter pylori colonization on the development of allergen-induced asthma.
TITLE: Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells
AUTHOR CONTACT:
Anne Müller
University of Zürich, Zürich, Switzerland.
Phone: 41.44.635.3474; Fax: 41.44.635.3484; E-mail: mueller@imcr.uzh.ch.
Christian Taube
Johannes Gutenberg University, Mainz, Germany.
Phone: 49.6131.176848; Fax: 49.6131.176668; E-mail: taube@3-med.klinik.uni-mainz.de.
View this article at: http://www.jci.org/articles/view/45041?key=75a3d98b6d6b62150d82
ALLERGY AND ASTHMA: New axis of evil in asthma
Asthma is an extremely common inflammatory disease of the airways. Research performed in mice, by Stephen Galli, Mang Yu, and colleagues, at Stanford University School of Medicine, Stanford, has now identified a new aspect to the immune response underlying this condition. If the results hold true in humans, these data suggest a new therapeutic target.
An allergic asthma attack occurs when the immune system mistakenly identifies a harmless substance, such as cat dander, as a dangerous invader. The immune response associated with allergic asthma is classified as a Th2 response. However, recent data have suggested that this might be too simplistic a classification, as they have implicated the Th1 cell–associated soluble factor IFN-gamma as having a role. Galli, Yu, and colleagues confirmed that IFN-gamma has a role in a mouse model of chronic asthma that exhibits many features of severe asthma in humans. They also identified what it does: it triggers the activation of immune cells known as mast cells, which are critical for the development of asthma. These data indicate that targeting IFN-gamma could provide a new therapeutic approach to treating asthma if the same IFN-gamma/mast cell axis has a role in the human condition.
TITLE: Identification of an IFN-gamma/mast cell axis in a mouse model of chronic asthma
AUTHOR CONTACT:
Stephen J. Galli
Stanford University School of Medicine, Stanford, California, USA.
Phone: 650.723.7975; Fax: 650.725.6902; E-mail: sgalli@stanford.edu.
Mang Yu
Stanford University School of Medicine, Stanford, California, USA.
Phone: 650.736.0069; Fax: 650.736.0073; E-mail: yumyu@stanford.edu.
View this article at: http://www.jci.org/articles/view/43598?key=40c2869f5f67c2e18553
MUSCLE BIOLOGY: Micro(RNA)managing muscle disease
Centronuclear myopathies are a group of congenital muscular diseases characterized by muscle weakness and abnormal localization of the nucleus in skeletal muscle cells. A team of investigators, led by Eric Olson and Ning Liu, at The University of Texas Southwestern Medical Center, Dallas, has now identified in mice a new molecular regulator of adult skeletal muscle structure and function that seems to act as a modulator of centronuclear myopathy.
MicroRNAs are small RNA molecules that regulate many biological processes by inhibiting the expression of specific genes. Olson, Liu, and colleagues found that mice lacking microRNAs miR-133a-1 and miR-133a-2 develop skeletal muscle abnormalities reminiscent of human centronuclear myopathies. They therefore conclude that, at least in mice, microRNAs miR-133a-1 and miR-133a-2 are essential for multiple facets of skeletal muscle function and maintenance. They further suggest that miR-133a could play a modulatory role in human centronuclear myopathies and that it might be possible to alter disease progression via effects on miR133a.
TITLE: Mice lacking microRNA 133a develop dynamin 2–dependent centronuclear myopathy
AUTHOR CONTACT:
Eric N. Olson
The University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Phone: 214.648.1187; Fax: 214.648.1196; E-mail: Eric.Olson@utsouthwestern.edu.
Ning Liu
The University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Phone: 214.648.1187; Fax: 214.648.1196; E-mail: Ning.Liu@utsouthwestern.edu.
View this article at: http://www.jci.org/articles/view/46267?key=992aa0c264151b27c743
###
END