(Press-News.org) SAN DIEGO, CA and PHILADELPHIA, PA – September 18, 2011 —Tarsa Therapeutics today presented positive safety and efficacy data from its Phase III ORACAL trial of OSTORA™, the company's oral recombinant salmon calcitonin tablet in development for the treatment of postmenopausal osteoporosis. These data were presented at the American Society for Bone and Mineral Research 2011 Annual Meeting by ORACAL investigator Neil Binkley, MD, who is an Associate Professor of Endocrinology and Geriatrics at the University of Wisconsin School of Medicine and Public Health in Madison, Wisconsin.
The ORACAL study was a Phase III multinational, randomized, double-blind, double-dummy, placebo-controlled trial of Tarsa's oral recombinant salmon calcitonin compared to commercially available, synthetic salmon calcitonin administered by nasal spray and to placebo. The trial enrolled 565 postmenopausal women in six countries with established osteoporosis.
Earlier this year, Tarsa reported top-line efficacy and safety results from the ORACAL trial. The data reported today show that OSTORA achieved all of the efficacy endpoints in the trial and indicate that the safety profile of OSTORA did not substantially differ from nasal calcitonin or placebo. The majority of adverse events were mild or moderate, and OSTORA was also significantly less immunogenic than nasal calcitonin spray.
"These are encouraging results that may be of particular relevance for the women with osteoporosis who are reluctant to take or unable to tolerate currently available treatments," said Dr. Binkley. "Potential safety issues with current drug therapies, while rare, have led some osteoporosis patients to discontinue therapy or avoid beginning treatment. Calcitonin has a long history of safety for postmenopausal osteoporosis treatment, and potential availability of this once-daily oral formulation could make it a useful new option for osteoporosis patients and their physicians."
According to the International Osteoporosis Foundation, studies indicate that up to half of osteoporosis patients stop their treatment after just one year. In one study, two-thirds of osteoporosis patients said their medication interfered with their lives in some way and over 20% of the women who missed or stopped their treatment said it was due to side effects.
"The encouraging efficacy and safety data presented at the ASBMR meeting, including data showing that OSTORA demonstrated superior efficacy to nasal calcitonin, reinforces our view that OSTORA has the potential to address an unmet need in current therapy," noted David Krause, MD, Chief Medical Officer of Tarsa. "Data suggest that approximately two million women in the U.S. alone have discontinued osteoporosis treatment for various reasons, which include poor tolerance or the perceived risk of adverse effects, leaving them more vulnerable to potentially disabling fractures. Reports from the field suggest many other newly diagnosed osteoporosis patients have not started drug treatment as a result of safety concerns. The data presented today indicate that once-daily OSTORA may have the potential to provide an effective, safe and convenient new option for these millions of women and their physicians."
Calcitonin is approved for the treatment of postmenopausal osteoporosis, but its use has been limited by the fact that it is currently available only in intranasal and injectable forms. Prior clinical studies show that Tarsa's oral calcitonin tablet delivers calcitonin effectively and reduces blood levels of bone resorption biomarkers.
"We view the new OSTORA data presented today as very promising news for osteoporosis patients and their physicians seeking additional treatment options," said David Brand, President and CEO of Tarsa. "We look forward to filing a New Drug Application for OSTORA with the Food and Drug Administration and to submitting a Marketing Authorization Application to the European Medicines Agency thereafter. We also are advancing our ongoing Phase Il trial in osteoporosis prevention, an indication for which our oral calcitonin could be especially relevant."
Tarsa's oral calcitonin is also being assessed in a double-blind Phase II study comparing the OSTORA tablet to placebo in postmenopausal women who have low bone mass (osteopenia) and are at increased risk of fracture. This proof-of-concept study is evaluating the ability of oral calcitonin to prevent osteoporosis and maintain bone mass in this population.
INFORMATION:
Tarsa is developing its OSTORA oral calcitonin under a licensing agreement with Unigene Laboratories that provides Tarsa with exclusive development and worldwide commercialization rights to Unigene's oral calcitonin product, with the exception of China. For more information on Unigene, see www.unigene.com.
The American Society for Bone and Mineral Research 2011 Annual Meeting, the premier scientific meeting discussing the latest concepts and innovations in bone and mineral research, is being held September 16-20, 2011 in San Diego, California. For more information, visit www.asbmr.org/Meetings/AnnualMeeting.aspx.
"Results of the ORACAL Trial: A Phase 3 Randomized Trial of the Safety and Efficacy of Orally Administered Recombinant Salmon Calcitonin Tablets in Postmenopausal Women with Osteoporosis," Neil Binkley MD, Michael Bolognese MD, James P Gilligan PhD, David S Krause MD. Presented at the ASBMR 2011 Annual Meeting, Oral Session 23, 2:30pm PDT, September 18, 2011.
*The International Osteoporosis Foundation. Osteoporosis: The Inside Story. Fact sheet. Retrieved August 29, 2011, from http://www.iofbonehealth.org/download/osteofound/filemanager/health_professionals/pdf/staying-power-material/doctor-questions-guide.pdf
About Tarsa Therapeutics
Tarsa Therapeutics is a venture-backed clinical stage biotechnology company developing OSTORA™, an oral formulation of calcitonin for the treatment and prevention of postmenopausal osteoporosis. Calcitonin has a long history of safety and efficacy, and availability of an oral form is expected to generate wider use. Tarsa recently reported positive efficacy and safety results from the Phase III ORACAL trial of its OSTORA tablet in the treatment of postmenopausal osteoporosis, and a Phase II osteoporosis prevention trial is underway. Tarsa is based in Philadelphia, PA. For more information, visit www.tarsatherapeutics.com.
END
VIDEO:
Receptors on the cell's surface crowd around the nanotube, effectively standing it upright. The cell mistakes the tube for a sphere and begins to engulf it.
Click here for more information.
PROVIDENCE, R.I. [Brown University] — It's been long known that asbestos spells trouble for human cells. Scientists have seen cells stabbed with spiky, long asbestos fibers, and the image is gory: Part of the fiber is protruding from the cell, like a quivering arrow that's found ...
Researchers have developed a new method to sequence and analyze the dark matter of life—the genomes of thousands of bacteria species previously beyond scientists' reach, from microorganisms that produce antibiotics and biofuels to microbes living in the human body.
Scientists from UC San Diego, the J. Craig Venter Institute and Illumina Inc., published their findings in the Sept. 18 online issue of the journal Nature Biotechnology. The breakthrough will enable researchers to assemble virtually complete genomes from DNA extracted from a single bacterial cell. By contrast, ...
BOULDER -- The planet's deep oceans at times may absorb enough heat to flatten the rate of global warming for periods of as long as a decade even in the midst of longer-term warming, according to a new analysis led by the National Center for Atmospheric Research (NCAR).
The study, based on computer simulations of global climate, points to ocean layers deeper than 1,000 feet (300 meters) as the main location of the "missing heat" during periods such as the past decade when global air temperatures showed little trend. The findings also suggest that several more intervals ...
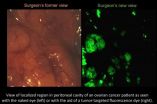
WEST LAFAYETTE, Ind. - The first fluorescence-guided surgery on an ovarian cancer patient was performed using a cancer cell "homing device" and imaging agent created by a Purdue University researcher.
The surgery was one of 10 performed as part of the first phase of a clinical trial to evaluate a new technology to aid surgeons in the removal of malignant tissue from ovarian cancer patients. The method illuminates cancer cells to help surgeons identify and remove smaller tumors that could otherwise be missed.
Philip Low, the Ralph C. Corely Distinguished Professor of ...
Scientists at the University of Plymouth have shown, for the first time in an animal, that nanoparticles have a detrimental effect on the brain and other parts of the central nervous system.
They subjected rainbow trout to titanium oxide nanoparticles which are widely used as a whitening agent in many products including paints, some personal care products, and with applications being considered for the food industry. They found that the particles caused vacuoles (holes) to form in parts of the brain and for nerve cells in the brain to die. Although some effects of nanoparticles ...
Production of crop milk, a secretion from the crops of parent birds, is rare among birds and, apart from pigeons, is only found in flamingos and male emperor penguins. Essential for the growth and development of the young pigeon squab, pigeon 'milk' is produced by both parents from fluid-filled cells lining the crop that are rich in fat and protein. Research published in BioMed Central's open access journal BMC Genomics uses new technology to study the genes and proteins involved in pigeon 'milk' production and shows that pigeon 'milk' contains antioxidants and immune-enhancing ...
Researchers whose findings on the detrimental impact of some common medicines on elderly people were widely reported earlier in the summer have found that taking a few of these medicines does not appear to cause further cognitive impairment in those already suffering from dementia.
In a paper published today by the journal Age and Ageing, Dr Chris Fox of the University of East Anglia (UEA) and colleagues from a number of other universities and the NHS describe how they studied a clinically representative sample of 224 people with established Alzheimer's dementia who ...
ITHACA, N.Y. – With an eye toward understanding DNA replication, Cornell researchers have learned how a helicase enzyme works to actually unzip the two strands of DNA. (Nature, online Sept. 18, 2011.)
At the heart of many metabolic processes, including DNA replication, are enzymes called helicases. Acting like motors, these proteins travel along one side of double-stranded DNA, prompting the strands to "zip" apart.
What had been a mystery was the exact mechanics of this vital biological process – how individual helicase subunits coordinate and physically cause the unzipping ...
Gamers have solved the structure of a retrovirus enzyme whose configuration had stumped scientists for more than a decade. The gamers achieved their discovery by playing Foldit, an online game that allows players to collaborate and compete in predicting the structure of protein molecules.
After scientists repeatedly failed to piece together the structure of a protein-cutting enzyme from an AIDS-like virus, they called in the Foldit players. The scientists challenged the gamers to produce an accurate model of the enzyme. They did it in only three weeks.
This class ...
TORONTO, ON – Researchers from the University of Toronto (U of T), the King Abdullah University of Science & Technology (KAUST) and Pennsylvania State University (Penn State) have created the most efficient solar cell ever made based on collodial-quatum-dots (CQD).
The discovery is reported in the latest issue of Nature Materials.
Quantum dots are nanoscale semiconductors that capture light and convert it into an energy source. Because of their small scale, the dots can be sprayed on to flexible surfaces, including plastics. This enables the production of solar cells ...