(Press-News.org) Empagliflozin (trade name Jardiance) has been approved since May 2014 for adults with type 2 diabetes mellitus in whom diet and exercise alone do not provide adequate glycaemic control. The German Institute for Quality and Efficiency in Health Care (IQWiG) examined in a dossier assessment whether the drug offers an added benefit over the appropriate comparator therapies in these patient groups.
According to the findings, such an added benefit is not proven: For four of five research questions, the manufacturer presented no relevant data in its dossier. For the fifth research question, on the one hand, it presented data from a direct comparison, in which empagliflozin was initially administered at a larger dose than recommended by the approval. Moreover, the study arms not only differed in the drug combination, but also in the therapeutic strategy. On the other hand, the manufacturer conducted two indirect comparisons based on an incomplete study pool and on studies that were unsuitable for the assessment.
Subindications Result in Five Research Questions
Empagliflozin is approved as monotherapy for patients who do not tolerate metformin. It is approved as add-on therapy in combination with other blood-glucose lowering drugs including insulin when these, together with diet and exercise, do not provide adequate glycaemic control.
The Federal Joint Committee (G-BA) specified different appropriate comparator therapies for the subindications, resulting in a total of five comparisons: empagliflozin as monotherapy versus a sulfonylurea (A), in combination with metformin versus metformin and a sulfonylurea (B1), in combination with another blood-glucose lowering drug also in comparison with metformin and sulfonylurea (B2), in combination with at least two other blood-glucose lowering drugs in comparison with metformin and human insulin (C) and in combination with insulin also in comparison with metformin plus human insulin (D).
For Four Research Questions, No Relevant Data Were Submitted
The manufacturer postulated an added benefit, which was partly considerable and partly non-quantifiable, for the research questions A, B2, C and D, but submitted no relevant data. An added benefit of empagliflozin versus the appropriate comparator therapies is therefore not proven in these cases.
Strict Target Levels Only in Comparator Arm
The manufacturer used one direct and two indirect comparisons to answer research question B1. In Study 1245.28, it compared empagliflozin with the sulfonylurea glimepiride. However, patients in the comparator arm received 1 to 4 mg glimepiride, without having sufficient flexibility, based on uniform HbA1c target levels. Dosing in the empagliflozin arm, in contrast, was consistently 25 mg daily. Hence the comparison not only referred to two drugs, but additionally to two therapeutic strategies.
In the first phase of the two-year study, blood glucose levels in the comparator arm decreased more rapidly and many more hypoglycaemias occurred than in the empagliflozin arm. More hypoglycaemias were also recorded in the glimepiride in the second half of the study, but it cannot be excluded that these hypoglycaemias also included events that were caused by the different therapeutic strategies.
Starting Dose Too High
In addition, the constant administration of 25 mg empagliflozin in the study is equivalent to 2.5 times the starting dose recommended in the approval. The blood-glucose lowering effectiveness of 10 mg empagliflozin cannot be assessed from the study.
Overall, the results of Study 1245.28 could not be interpreted with sufficient certainty. Regardless of this, the study showed no overall advantage of empagliflozin because although there were fewer hypoglycaemias under empagliflozin, there were also more genital infections and renal and urinary disorders as well as generally more serious adverse events than under glimepiride.
Indirect Comparisons Also not Informative
In the first of the two indirect comparisons, empagliflozin 25 mg plus metformin was the so-called common comparator, which was compared with empagliflozin 10 mg plus metformin in Study 1275.1, and with glimepiride 1 to 4 mg plus metformin in the aforementioned Study 1245.28. However, the manufacturer did not consider Study 1245.23/1245.31, which was also relevant. In addition, the comparison of two treatment regimens in Study 1245.28 made it impossible to clearly attribute the effect to the drug.
In the second indirect comparison, the pharmaceutical company also used a study that was unsuitable for the assessment because different treatment regimens were used in the two study arms with a target blood glucose level being specified only in the comparator arm. Data from the same study were already submitted in a dossier on linagliptin, for which also no added benefit is proven for this reason, among others. IQWiG therefore concluded: An added benefit of empagliflozin is not proven.
G-BA Decides on the Extent of Added Benefit
The dossier assessment is part of the overall procedure for early benefit assessments according to the Act on the Reform of the Market for Medicinal Products (AMNOG) supervised by the G-BA. After publication of the manufacturer's dossier and IQWiG's assessment, the G-BA conducts a commenting procedure, which may provide further information and result in a change to the benefit assessment. The G-BA then decides on the extent of the added benefit, thus completing the early benefit assessment.
INFORMATION:
An overview of the results of IQWiG's benefit assessment is given by a German-language executive summary. In addition, the website » http://www.gesundheitsinformation.de, published by IQWiG, provides easily understandable and brief German-language information on Empagliflozin.
The G-BA website contains both general English-language information on benefit assessment pursuant to §35a Social Code Book (SGB) V and specific German-language information on the assessment of empagliflozin.
More English-language information will be available soon (Sections 2.1 to 2.6 of the dossier assessment as well as subsequently published health information on » http://www.informedhealthonline.org). If you would like to be informed when these documents are available, please send an e-mail to » info@iqwig.de.
Following a 5,000 km long ocean survey, research published in the Proceedings of the National Academy of Sciences presents a new way to measure how the acidification of water is affecting marine ecosystems over an entire oceanic basin.
As a result of man-made emissions, the content of CO2 in the atmosphere and oceans has increased dramatically during recent decades. In the ocean, the accumulating CO2 is gradually acidifying the surface waters, making it harder for shelled organisms like corals (Figure 1) and certain open sea plankton to build their calcium carbonate ...
Flexible electronic sensors based on paper -- an inexpensive material -- have the potential to some day cut the price of a wide range of medical tools, from helpful robots to diagnostic tests. Scientists have now developed a fast, low-cost way of making these sensors by directly printing conductive ink on paper. They published their advance in the journal ACS Applied Materials & Interfaces.
Anming Hu and colleagues point out that because paper is available worldwide at low cost, it makes an excellent surface for lightweight, foldable electronics that could be made and ...
Now that car makers have demonstrated through hybrid vehicle success that consumers want less-polluting tailpipes, they are shifting even greener. In 2015, Toyota will roll out the first hydrogen fuel-cell car for personal use that emits only water. An article in Chemical & Engineering News (C&EN), the weekly newsmagazine of the American Chemical Society, explains how hydrogen could supplant hybrid and electric car technology -- and someday, even spur the demise of the gasoline engine.
Melody M. Bomgardner, a senior editor at C&EN, notes that the first fuel-cell vehicles ...
Exposure to peanut proteins in household dust may be a trigger of peanut allergy, according to a study published today in the Journal of Allergy and Clinical Immunology.
The study was conducted in 359 children aged 3-15 months taking part in the NIH-sponsored Consortium for Food Allergy Research (CoFAR) study. These children were at high risk of developing a peanut allergy based on having likely milk or egg allergy or eczema. The study found that the risk of having strong positive allergy tests to peanut increased with increasingly higher amounts of peanut found in ...
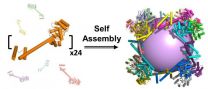
UCLA biochemists have created the largest-ever protein that self-assembles into a molecular "cage." The research could lead to synthetic vaccines that protect people from the flu, HIV and other diseases.
At a size hundreds of times smaller than a human cell, it also could lead to new methods of delivering pharmaceuticals inside of cells, or to the creation of new nanoscale materials.
The protein assembly, which is shaped like a cube, was constructed from 24 copies of a protein designed in the laboratory of Todd Yeates, a UCLA professor of chemistry and biochemistry. ...
A new treatment for Marfan syndrome, a rare genetic disease that can lead to heart problems, works as well as the currently recommended medical therapy, beta blockers, according to an article in the New England Journal of Medicine.
Angela Sharkey, M.D., professor of pediatrics at Saint Louis University, and a study author, said researchers found losartan, which had been more effective in an animal model of Marfan syndrome, was equally effective to a high dose of the beta blocker atenolol.
"While there may be certain patients who respond better to one drug or another, ...
Researchers from the University of Melbourne found unlike other laser treatments, this new faster laser did not result in damage to the retina, the sensitive light detecting tissue at the back of the eye.
Associate Professor Erica Fletcher from the Department of Anatomy and Neuroscience said this was the first report detailing how this new laser treatment may improve eye health in those with AMD. In the early stages, the disease is characterised by the presence of small fatty deposits called drusen and thickening in a membrane at the back of the eye.
Published this ...
Barcelona, Spain: A new drug that targets not only common cancer-causing genetic mutations in patients with non-small cell lung cancer (NSCLC), but also a form of the mutation that causes resistance to treatment, has shown promising results in patients in a phase I/II clinical trial. The research will be presented today (Friday) at the 26th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics in Barcelona, Spain.
Approximately 10-15% of Caucasian and 30-35% of Asian patients with NSCLC have a mutation in the epidermal growth factor receptor (EGFR), which ...
If it's help a woman needs, maybe she should wear high heels. That's the message from Nicolas Guéguen of the Université de Bretagne-Sud in France, after he observed how helpful men are towards women in high heels versus those wearing flat, sensible shoes. The study, published in Springer's journal Archives of Sexual Behavior, is the first ever to investigate how the height of a woman's shoe heel influences how men behave towards her.
Research across various cultures has shown at length how important physical features, such as body size and the style and color ...
Approximately 40 percent of the earth's surface is covered by drylands in which average annual precipitation is lower than evaporation. The changes projected to unfold in these areas in the course of climate change are alarming. Greater variations in annual and seasonal precipitation will lead to more frequent droughts and, presumably, longer drought periods. This means that drylands are among those areas most severely affected by climate change.
Research has thus far not adequately addressed the question of how strongly annual plant growth in pasture landscapes - hence ...