It is hoped that these research results can be used in potato breeding as a basis for suppressing the synthetization of poisonous compounds.
These research results were published in the international academic journal 'Nature Communications' on February 26.
Main Points
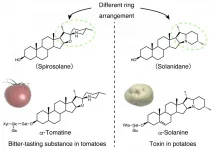
α-solanine is a toxic steroidal glycoalkaloid (SGA) (*1) found in potatoes. Tomato's α-tomatine is astringent-tasting SGA that accumulates inside unripe fruits. Based on their chemical structures, SGAs can be divided into two general classes, solanidanes (*2, e.g.α-solanine) and spirosolanes (*3, e.g. α-tomatine). The research group revealed that the toxic α-solanine in potatoes is biosynthesized from spirosolane. They discovered that the dioxygenase DPS (*4) is the key enzyme for this catalytic conversion. It was also revealed that the α-solanine biosynthesis pathway in potatoes diverged from the spirosolane biosynthesis pathway due to the evolution of DPS. Research Background
α-solanine is a type of toxic steroidal glycoalkaloid (SGA), which accumulates in the green skin on potato tubers (*5) and in tuber sprouts. SGA is not only found in potatoes but also in other plants of the Solanaceae family, including crops like tomatoes and eggplants. These substances are poisonous to many living things and serve as one of the plants' natural defenses. Low concentrations of SGA in potatoes cause a bitter taste and larger amounts can cause food poisoning. For this reason, biosynthesis research has been conducted with the aim of controlling the accumulation of SGA in potatoes.
Based on their skeletal chemical structures, SGAs can be divided into two general classes, solanidanes and spirosolanes (Figure 1). The potato toxinα-solanine is an example of a solanidane, whereas α-tomatine, which accumulates inside unripe tomatoes, is a spirosolane. It is known that both classes of SGA are biosynthesized from cholesterol. Up until now, several genes that encode the catalytic enzymes in SGA biosynthesis have been discovered and potato and tomato plants share these enzymes in the common pathway of SGA biosynthesis. However, the steps and enzymes involved in the metabolic branch point between solanidane- skeleton and spirosolane-skeleton formation remains an unsolved mystery.
This research group showed that the potato toxinα-solanine is biosynthesized from spirosolane. In a world first, they discovered that the dioxygenase DPS is the key to this conversion.
Research Findings
Potatoes contain the toxic solanidanes α-solanine and α-chaconine. The research group investigated theα-solanine biosynthesis pathway in potato plants. Using genome editing, they disrupted the biosynthetic enzyme gene in potato so that it was unable to produceα-solanine. Feeding α-tomatine (a spirosolane found in tomatoes) to the disruptant resulted in a metabolic conversion to the corresponding solanidane compound. In addition, it was found that this metabolic alteration could be suppressed with a 2-oxoglutarate dependent dioxygenase inhibitor, revealing that a dioxygenase is responsible for the oxidation reaction that synthesizes solanidanes from spirosolanes.
The researchers singled out a 2-oxoglutarate dependent dioxygenase (DPS) gene that was expressed in potato during α-solanine synthesis. To investigate this further, the researchers generated modified plants in which DPS gene expression was suppressed via RNA interference (*7). The solanidane concentrations in these modified potato plants were far lower than in the unmodified group, and spirosolanes accumulated inside the plants in place of solanidanes. Next, the researchers measured the enzymatic activity of DPS by recombining the proteins and expressing them in E. coli. The results revealed the unique catalytic role of DPS in spirosolane's conversion into solanidane (Figure 2). This proved that DPS is the key enzyme responsible for this conversion.
This research revealed that the potato's ability to produce α-solanine came about due to the evolution of DPS, which is responsible for metabolically converting spirosolanes (e.g. α-tomatine) into solanidanes. It is known that tomatoes also have an enzyme for metabolizing spirosolanes. The bitter-tasting α-tomatine is found in unripe tomatoes but is metabolized into the tasteless, non-toxic esculeoside A as the fruits ripen. The catalyst for this reaction is 23DOX (*8), which is also a dioxygenase.
From the relationship between their chromosomal positions and phylogenetic analysis, it was revealed that the α-solanine biosynthase DPS has evolved from the same precursor gene as the non-toxic α-tomatine's catalytic enzyme 23DOX. Thus, it is believed that the evolution of the dioxygenase gene that metabolizes spirosolanes is one of the main drivers of the development of structural and functional variation in SGAs.
Further Developments
Potatoes have been termed a potentially dangerous food because large concentrations of toxic α-solanine can cause food poisoning. It is hoped that these research results can provide a basis for future potato varieties in which the biosynthesis of toxic compounds is suppressed by targeting the DPS gene.
As shown in this research, the evolutionary origins of the structural diversity of SGAs provide clues towards discovering unknown SGA synthesis enzymes involved in biological functions in various plants. Illuminating these functions could pave the way for the molecular breeding of plant varieties that are able to adapt to different stressful environments.
INFORMATION:
Glossary
1. Steroidal Glycoalkaloid (SGA): An SGA consists of alkaloids containing nitrogen atoms arranged in a skeletal steroid structure. It is a toxic alkaloid glycoside with an oligosaccharide attached to the hydroxyl group at position C3 of the steroid. SGAs are secondary metabolites that accumulate in plants of the Solanaceae family. The SGAs α-solanine and α-chaconine are found in potatoes and are known to cause food poisoning.
2. Solanidanes: The skeletal steroid structure of some SGA chemical compounds, such as α-solanine in potatoes.
3. Spirosolanes: The skeletal steroid structure of some SGA chemical compounds, such as α-tomatine in tomatoes.
4. DPS: DPS stands for Dioxygenase for Potato Solanidane synthesis. It is the key enzyme for the biosynthesis ofα-solanine in potatoes. DPS is the catalytic enzyme for the metabolic alteration of spirosolane to solanidane by C-16 hydroxylation.
5. Tuber: An enlarged underground structure in some plant species that stores nutrients. Plants with edible tubers include potato, konnyaku and Jerusalem artichoke.
6. 2-oxoglutarate dependent dioxygenase: 2-oxoglutarate dependent dioxygenases are a super family of enzymes that are water-soluble and play various roles in many biological processes. They require 2-oxoglutarate (α-ketoglutarate) and O2 to hydrate substrates.
7. RNA interference: RNA interference is a phenomenon where an antisense RNA strand, which is complimentary to a specific gene's mRNA, and a sense RNA strand become a double-stranded RNA, suppressing the expression of the gene. This knowledge forms the basis of a method to control the expression of target genes in experiments.
8. 23DOX: This enzyme hydrolyzes spirosolane at C-23. It is part of the 2-oxoglutarate dependent dioxygenase super family of enzymes. In tomato plants, it hydrolyzes the bitter-tasting SGA α-tomatine at C-23.
Acknowledgements
This research was partially funded by the following:
A Grant-in-Aid for JSPS Fellows for the research project entitled 'The chemical evolution of steroidal glycoalkaloids in Solanaceae' (grant number JP19J10750, recipient: Akiyama Ryota).
The Japanese Ministry of Agriculture, Forestry and Fisheries' 'Development of new varieties and breeding materials in crops by genome editing' program for advancing research.
The Cross-ministerial Strategic Innovation Promotion (SIP) Program.
Journal Information:
Title:
"The biosynthetic pathway of potato solanidanes diverged from that of spirosolanes due to evolution of a dioxygenase"
DOI: 10.1038/s41467-021-21546-0
Authors:
Ryota Akiyama1, Bunta Watanabe2, Masaru Nakayasu1†, Hyoung Jae Lee1, Junpei Kato1, Naoyuki Umemoto3, Toshiya Muranaka4, Kazuki Saito3,5, Yukihiro Sugimoto1, Masaharu Mizutani1
1. Graduate School of Agricultural Science, Kobe University.
2. Institute for Chemical Research, Kyoto University.
3. RIKEN Center for Sustainable Resource Science.
4. Department of Biotechnology, Graduate School of Engineering, Osaka University.
5. Graduate School of Pharmaceutical Sciences, Chiba University.
†Currently a graduate student at the Research Institute for Sustainable Humanosphere, Kyoto University.
Journal:
Nature Communications