(Press-News.org) Charcot Marie Tooth and Dejerine-Sottas syndrome are groups of diseases that involve the breakdown of the myelin sheath covering nerve axons.
As this myelin sheath breaks down, people who have these disorders suffer nerve damage in the arms and legs--those with Dejerine-Sottas disease may never walk or may lose the ability to walk by the time they are teenagers.
Researchers have known that a protein called PMP22, which is important for nerve myelin, is likely involved in the disease. But because the protein is so small and part of the cell membrane, it's difficult to study. Now, an interdisciplinary team from the University of Michigan and Vanderbilt University have used a cutting-edge technique called ion mobility-mass spectrometry (IM-MS) to show that an unstable, mutant version of the PMP22 protein associates with another mutant PMP22, forming a stable complex called a "dimer."
"If you get one of these mutations in your PMP22 gene, that leads to the creation of this mutant protein. Then, our results indicate that the mutant protein produced is likely going to dimerize," said Brandon Ruotolo, lead author on the new study and a professor of chemistry at U-M. "Because it's stable, it's not going to re-form into monomeric protein that is then going to give you normal myelin sheath production."
Their results, published in the Proceedings of the National Academy of Sciences, provide a new view into how these diseases work, and could one day provide a target for therapeutics.
Vanderbilt researcher Charles Sanders began studying PMP22 in 2000. It was a protein known to be involved in these neurological diseases, but few were studying it. There are other diseases related to problems with protein folding, Sanders says. Typically, misfolding proteins cause disease in two ways: one is that when they misfold, they are degraded and can't complete the job they're meant to do. A second is that a protein clumps up, gumming up the cell and preventing it from doing its job properly.
"With PMP22, it's never been completely clear how it causes disease. It's definitely related to problems with folding, but how does this really work? What Brandon's results suggest is that when this protein is unstable, it doesn't fold correctly, but instead of getting degraded, it forms this misfolded dimer," Sanders said. "We still don't know exactly what it then does to cause these diseases, but it's an interesting and unusual observation that makes these diseases different from all of the other protein-folding diseases."
Over the years, Sanders' lab has done a series of measurements to quantitate the stability of mutant forms of PMP22. His lab has also collaborated with U-M researcher Melanie Ohi to study the healthy function of PMP22, work that started while Ohi was on the faculty at Vanderbilt.
"We were able to see what we called myelin-like assemblies that allowed us to get some idea of what this protein might actually be doing in the context of a membrane. When we came to U-M, our goal was to get a high-resolution structure, which is really hard because this is a really small protein," Ohi said. "We needed to figure out if the protein Dr. Yadav was making for structural studies was forming larger oligomers and, surprisingly, PMP22 works well for Brandon's technique."
Currently an associate professor in U-M's Life Sciences Institute, Ohi met Ruotolo, whose lab specializes in using IM-MS to capture native structures of biomolecules as well as how they assemble.
To view these proteins using this kind of mass spectrometry, Ruotolo's lab transfers the protein into the gas phase using a technique called nano-electrospray ionization. The technique works by aersolzing protein-containing solutions into tiny charged droplets. The solvent carrying the protein quickly dries--this process happens over just milliseconds--which leaves the charged protein behind. These charged proteins are then drawn into a vacuum environment inside the mass spectrometry instrument.
Once inside the instrument, ions encounter a chamber pressurized with a small amount of background gas. Within this chamber, the instrument uses an electric field to pull the ionized proteins through a series of electrodes in the presence of the background gas. This separates the ions according to their size. A subsequent device uses a chamber operated under strict vacuum conditions to separate the same ions according to their mass. This way, the researchers are able to view both the structures of the proteins, and separate PMP22 dimers from monomers in order to evaluate them independently.
The researchers then heat the ionized proteins and measure their size, repeating this step several times in order to measure the changes in size as the proteins unfold. This tells the researchers how stable the proteins are--and in particular, the stability of the misfolded dimer proteins.
The researchers say developing therapeutics to interfere with these mutants clumping together is further down the road, but knowing how and where the protein self-associates provides a road map for a new venue of research.
"Not only is this a big step forward in what we know about the protein, mass spectrometry has such fabulous capabilities that this opens the door for a whole unbelievable set of things that we could do in the future with this protein," Sanders said. "With the sort of techniques that Brandon works with, you could imagine eventually extending this to drug discovery, using this basic method as an assay for finding compounds that correct these defects."
INFORMATION:
The study is titled "Ion Mobility Mass Spectrometry Reveals the Role of Peripheral Myelin Protein Dimers in Peripheral Neuropathy." PNAS will make it available at DOI:10.1073/pnas.2015331118.
Study co-authors include U-M chemistry graduate students Sarah Fantin, Kristine Parson and Brock Juliano; Life Sciences Institute senior research associate Pramod Yadav; and Vanderbilt research fellow Geoffrey Li.
Brandon Ruotolo
Melanie Ohi
Charles Sanders
Scientists have produced a comprehensive roadmap of muscle aging in mice that could be used to find treatments that prevent decline in muscle mobility and function, according to a report published today in eLife.
The study reveals which molecules in the muscle are most significantly altered at different life stages, and shows that a molecule called Klotho, when administered to mice in old, but not very old, age, was able to improve muscle strength.
Age-related loss of skeletal muscle mass and function - called sarcopenia - is associated with loss of mobility and increased risk of falls. Yet, although scientists know how sarcopenia affects the appearance and behaviour of muscle tissues, the underlying molecular mechanisms for sarcopenia remain poorly understood. Current treatments ...
Diodes are widely used electronic devices that act as one-way switches for current. A well-known example is the LED (light-emitting diode), but there is a special class of diodes designed to make use of the phenomenon known as “quantum tunneling”. Called resonant-tunneling diodes (RTDs), they are among the fastest semiconductor devices and are used in countless practical applications, such as high-frequency oscillators in the terahertz band, wave emitters, wave detectors, and logic gates, to take only a few examples. RTDs are also sensitive to light and can be used as photodetectors or optically active elements in optoelectronic circuits.
Quantum tunneling (or the tunnel ...
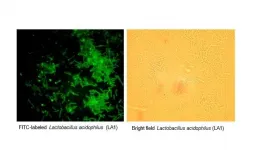
Philadelphia, April 20, 2021 - Intestinal epithelial tight junctions (TJs) act as a functional and structural barrier against harmful antigens that promote intestinal inflammation in inflammatory bowel disease (IBD) and other inflammatory conditions of the gut. A defective intestinal TJ barrier, sometimes known as "leaky gut," plays an important role in exacerbating and prolonging intestinal inflammation. New research reported in The American Journal of Pathology, published by Elsevier, shows that the probiotic Lactobacillus acidophilus (L. acidophilus) strain known as LA1 can generate a rapid and sustained enhancement of this defective intestinal barrier and effectively treat intestinal inflammation by preserving and restoring ...
Current national strategies for bridge maintenance favor replacement over maintenance. A fairly simple depreciation formula is used, resulting in overly conservative assessments of a bridge's long-term health. In a study published in the American Society of Civil Engineers' Journal of Performance of Constructed Facilities, researchers from UGA's College of Engineering propose a new model for the first time. This new approach considers the interaction of 60 to 80 bridge components in predicting long-term bridge performance and focuses on maintenance instead of replacement.
"Rather than considering a bridge as a monolithic structure, the bridge coactive model assesses a bridge as a system in which changes in the condition of each coactive element not only directly affects the ...
FAYETTEVILLE, Ark. - The fearsome tyrannosaur dinosaurs that ruled the northern hemisphere during the Late Cretaceous period (66-100 million years ago) may not have been solitary predators as popularly envisioned, but social carnivores similar to wolves, according to a new study.
The finding, based on research at a unique fossil bone site inside Utah's Grand Staircase-Escalante National Monument containing the remains of several dinosaurs of the same species, was made by a team of scientists including Celina Suarez, University of Arkansas associate professor of geosciences.
"This supports our hypothesis ...
TAMPA, Fla (April 20, 2021) — Chaperone protein imbalance can play a significant role in initiating toxic accumulation of tau in the aging brain - an early step in the development of Alzheimer's disease and related neurodegenerative disorders known as tauopathies, a new preclinical study by University of South Florida Health (USF Health) neuroscientists suggests.
In humans, misfolding of the protein tau leads to its toxic accumulation inside brain cells, the formation of these tau aggregates into hallmark neurofibrillary tangles, neuron death, and eventually symptoms of cognitive decline such as memory loss and diminished thinking skills.
In this study the ...
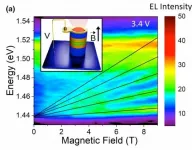
LOS ALAMOS, N.M., April 19, 2021 -- A recent series of experiments at the National High Magnetic Field Laboratory (National MagLab) at Los Alamos National Laboratory leveraged some of the nation's highest-powered nondestructive magnets to reveal an exotic new phase of matter at high magnetic fields. The experiments studied the unusual Kondo insulator ytterbium dodecaboride (or YbB12) and were the first results from the new 75-tesla duplex magnet housed at the National MagLab's Pulsed Field Facility at Los Alamos.
"This magnet and the resulting experiments are the first fruits of the National Science Foundation-supported pulsed magnet surge," said Michael Rabin, director of the Pulsed Field Facility at Los ...
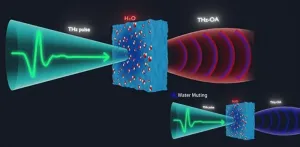
Radiation at terahertz frequencies (wavelengths between 0.03 and 0.3 mm) can be used successfully to analyze the structural dynamics of water and biomolecules. But applying the technique to aqueous solutions and tissues remains challenging, since terahertz (THz) radiation is strongly absorbed by water. While this absorption enables certain analyses, such as the structure of water and its interactions with biological solutes, it limits the thickness of samples that can be analyzed, and it drowns out weaker signals from biomolecules of interest. Strong absorption of THz ...
CORVALLIS, Ore. - Four decades after their capture more than a half-mile below the ocean's surface, three snailfish species have received their scientific names, two of them from school children on Guam in the island's native Chamorro language.
The rare specimens of liparids were collected in the early 1980s in traps set in the Mariana Archipelago in the western Pacific Ocean, deposited with NOAA's Pacific Islands Fisheries Science Center in Hawaii and did not get examined until recently, when they were noticed during the center's move to a new location. ...
As the back-to-school rush begins, podiatry experts at the University of South Australia are encouraging parents to get their children's school shoes professionally fitted, as new research confirms that ill-fitted footwear can significantly impede foot movement and comfort.
In a new study, researchers tested the effect of shoe size on foot motion and comfort among children aged 8 to 12 years, finding that shoes that were one size too small restricted the normal movement of the heel, arch and big toe joint during walking.
The study also confirmed that a comfortable shoe fit can be determined by a 'rule of thumb', where the wearer's thumb width from their longest toe to the end of their shoe is an effective and accurate measure ...