ß cells are critical guardians of the body’s metabolic balance. They are the only cells capable of producing insulin, which regulates blood sugar levels by designating dietary sugar for immediate use or storage.
In Type 1 diabetes, ß cells are attacked by the body’s own immune system, rendering them unable to produce insulin.
Type 2 diabetes arises from insulin resistance; the resulting excess blood sugar from a person’s diet causes ß cells in the pancreas to work overtime. Eventually, ß cells can no longer keep up and blood sugar concentrations can rise to dangerously high levels.
Both diseases are treated by enhancing insulin action, either by providing insulin itself, or by augmenting its activity and release into the blood. Some people with Type 1 diabetes may elect to have a ß cell transplant, an experimental procedure in which functioning cells from a donor are implanted into the pancreas.
The new findings, published in Cell Metabolism, suggest several potential paths that could inform future diabetes treatments, such as adjusting the ratio of ß cell subtypes in transplants to ensure optimal function.
“All cells vary in some way, but these two ß cell subtypes are discretely and consistently different from one another. This indicates that they serve two different but necessary functions as insulin producers. They are specialists, each with their own roles,” said J. Andrew Pospisilik, Ph.D., a Van Andel Institute professor and senior author of the study. “We also see differences in the ratio of one subtype to another in diabetes. Understanding these two cell types — and their relationship to each other — gives us a clearer picture of diabetes and offers new opportunities for treatment.”
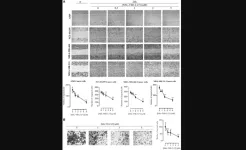
Scientists have long recognized differences among ß cells, but this study is the first to clearly delineate specific cell subtypes. The findings were identified in both mouse models and in human ß cell samples.
The two types — described by study authors as ßHI and ßLO — differ in specific function, size, shape, and epigenomic features, among other characteristics. They also exhibit contrasting patterns of surface markers, which help cells send and receive chemical messages. ßHI cells appear to be more prevalent in Type 2 diabetes.
Importantly, the subtypes can be separated by the presence or absence of a protein called CD24, which acts as a marker that allows targeting of one type and not the other. This distinction may inform development of more precise diabetes treatment strategies and offers a critical tool that enables diabetes researchers to better study each cell type in depth.
The findings also reshape what is known about how ß cells develop early in life. ß cells are among the longest-lived cells in the body, with lifespans of 30 to 40 years. Like all cells, the earliest ß cells arise from stem cells, which are blank slates that differentiate into the many cell types that comprise the body. This process is largely guided by specialized proteins called transcription factors, which switch genes “on” and “off.”
However, the study suggests ß cells may be an exception. The researchers identified epigenetic dosage rather than transcription factors as a driving force behind the decision of ß cells to be become ßHI or ßLO. This is the first time epigenetic dosage has been shown to change the ratio of related cell types.
Like transcription factors, epigenetic marks tell genes when to be active and when to be silent. Epigenetic dosage refers to the amount of these marks. In ß cells, the team previously identified an epigenetic mark called H3K27me3 as a key driver of differentiation. In this new study, they found that dosage of the same mark controls ßHI versus ßLO numbers and, as a result, offers a new target for potential new diabetes treatments.
“The beauty of this mechanism is its novelty — it’s purely driven by epigenetics with no help from transcription factors,” Pospisilik said. “The key here is that epigenetic changes can be reversed, which opens a whole host of questions with implications for treatment.”
Erez Dror, Ph.D., of Max Planck Institute of Immunobiology and Epigenetics, is the first author of this study.
Other authors include Luca Fagnocchi, Ph.D., Vanessa Wegert, M.Sc., Stefanos Apostle, M.S., Brooke Grimaldi, Ph.D., Tim Gruber, Ph.D., Illaria Panzeri, Ph.D., Ayush Semwal, M.S., Ben Johnson, Ph.D., Adelheid Lempradl, Ph.D., and Hui Shen, Ph.D., of VAI; Steffen Heyne, Ph.D., Kira Daniela Höffler, Victor Kreiner, Reagan Ching, Ph.D., and Tess Tsai-Hsiu Lu, Ph.D., of Max Planck Institute of Immunobiology and Epigenetics; Parijat Senapati, Ph.D., and Dustin Schones, Ph.D., of Beckman Research Institute of City of Hope; and Axel Imhof, Ph.D., of Biomedical Center Munich, Ludwig Maximilian University of Munich. Technical support was provided by the Max Planck Institute of Immunobiology and Epigenetics Cores and the following VAI Core Technologies and Services: Optical Imaging, Flow Cytometry, Genomics, and Bioinformatics and Biostatistics. The Alberta Diabetes Institute Islet Core and Clinical Islet Laboratory of the University of Alberta, in collaboration with the Human Organ Procurement and Exchange (HOPE) program and Trillium Gift of Life Network, provided the islet cells.
All donors’ families gave informed consent for the use of pancreatic tissue in research. Without them, this work would not have been possible.
Research reported in this publication was supported by Van Andel Institute; Max Planck Gesellschaf; the European Research Council under award nos. ERC-StG-281641 and ERC-CoG-682679 (Pospisilik); the European Foundation for the Study of Diabetes/Eli Lilly (Dror); the National Human Genome Research Institute of the National Institutes of Health under award no. R21HG011964 (Pospisilik); and the NIH Common Fund, through the Office of the NIH Director (OD), and the National Human Genome Research Institute of the National Institutes of Health under award no. R01HG012444 (Pospisilik and Nadeau). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding organizations.
###
ABOUT VAN ANDEL INSTITUTE
Van Andel Institute (VAI) is committed to improving the health and enhancing the lives of current and future generations through cutting-edge biomedical research and innovative educational offerings. Established in Grand Rapids, Michigan, in 1996 by the Van Andel family, VAI is now home to more than 500 scientists, educators and support staff, who work with a growing number of national and international collaborators to foster discovery. The Institute’s scientists study the origins of cancer, Parkinson’s and other diseases and translate their findings into breakthrough prevention and treatment strategies. Our educators develop inquiry-based approaches for K-12 education to help students and teachers prepare the next generation of problem-solvers, while our Graduate School offers a rigorous, research-intensive Ph.D. program in molecular and cellular biology. Learn more at vai.org.
END