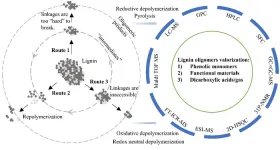
Lignin depolymerization is playing a pivotal role in transforming the second most abundant biopolymers in nature into many valuable chemicals/fuels. This route could directly replace their petrol-based equivalents and therefore a great pathway to fight climate change and contribute to future sustainability. Interpretation of the reaction pathways is always desired to gain an insightful mechanic view in understanding the depolymerization chemistry and also paving new paths for lignin valorization at the industrial scale. However, such interpretation heavily relies on the state-of-art analytical capability since in the process there are many oligomeric products formed but not well-characterized due to the limited instrumental analysis choices. A team of scientists, therefore, summarized the considerable progress in the formation and identification of lignin oligomeric products in recent years. Both the existing and expected potential valorization routes are discussed, and technical hurdles and recommendations are provided in an attempt to catalyze the development of new discoveries and enabling technologies. The work is published in Industrial Chemistry & Materials on Mar. 10, 2023.
"Analyzing the oligomeric products from lignin polymerization is playing important role in transforming the renewable lignin polymer to many valuable chemicals and fuels," said Seema Singh, a director of biomass deconstruction division at Joint Bioenergy Institute, "In this review, we systematically discussed the recent development of identification of the oligomeric products from various lignin depolymerization, thus revealing the mechanical insight of the processes and to provide helpful guidance for understanding the formation of such oligomers chemically. We also provided our perspectives on the existing and expected potential valorization routes for the oligomers as well as discussed the remaining challenges to this field."
Common targeted products from lignin depolymerization are monomeric phenols, aromatics, cycloalkanes, and dicarboxylic acids. The large scale of one such process can be exemplified by the industrial production of vanillin through oxidative catalytic depolymerization. The relatively low cost of the substrate and the ease of operation counteract the fact that the yield of vanillin is often not high, which overall still makes this process economically viable. The same logic, however, may not be well applied to other lignin-valorization routes, such as reductive catalytic depolymerization, which is often a pre-treatment of lignin (via hydrogenolysis) if the final products are fuel-ranged cycloalkanes. A similar dilemma also exists in the thermochemical treatment of lignin. Regardless of the chemical treatments, oligomeric fractions from any lignin depolymerization are inevitably formed as the side products, and in many cases, can be the major products as well. The inherent causes, although not very clear so far, have been extensively discussed in many studies. One of the commonly agreed viewpoints is the unavoidable participation of the crosslinking/repolymerization reactions during the proceeding of various lignin depolymerizations. Major efforts, therefore, had been made to mitigate the repolymerization processes.
"The depolymerization of lignin provides access to a new dimension of the utilization of lignin-based materials," said Blake Simmons, director of Biological System and Engineering Division at Lawrence Berkeley National Laboratory, "In order to improve processes of bioenergy/bioproducts, more research efforts should emphasize on the reactivity and selectivity of depolymerized lignin oligomers. There is huge room to control the structure, molecular weight, branching, and functional complexity to adapt lignin to various valorization processes."
"So far, we don’t know if repolymerization is the only explanation for the formation of the oligomeric products or not, but there have been enough publications, especially in recent years, reporting that the formation could be sometimes also due to other reasons depends on the condition," said Yinglei Han, a postdoctoral researcher at Joint Bioenergy Institute and Sandia National Laboratories, "we therefore highly motivated to summarize all the options and present an overview on elucidating the formation causes. Presently, three routes have been identified and established to reveal the formation of the oligomeric products during various depolymerization."
"One of the biggest technical hurdles in achieving this potential is the difficulty in identifying all depolymerization products," said Han, "The oligomers are indispensable pieces if one wants to gain an insightful mechanistic view of the process or to valorize them. Such difficulty could mainly be attributed to the structural heterogeneity of lignin which could significantly diversify the derived oligomeric products compared to the depolymerization of those petroleum-based polymers (e.g., polyethylene, polystyrene)."
The state-of-the-art methods for characterizing these oligomeric products are Two-dimensional Heteronuclear Single Quantum Coherence nuclear magnetic resonance, gel permeation chromatography, matrix-assisted laser desorption/ionization-time of flight mass spectrometry, LTQ Orbitrap mass spectrometry (Elite) and Fourier-transform ion cyclotron resonance mass spectrometry, combined with other less informative instrumental analyses, such as UV/vis, UV-florescence, pyrolysis gas chromatography mass spectrum, elemental analysis, thermogravimetric analysis, and Fourier-transform infrared spectroscopy. All these techniques combined, however, are still inadequate for distinguishing and then quantifying each oligomer. More efforts are needed in this aspect in the future, which is pivotal in utilizing these oligomeric products in many other applications.
"The reported pathways for the valorization of such oligomeric products can be generally categorized into three, 1) further depolymerization for more phenolic monomers, 2) modification of lignin oligomers into value-added materials, and 3) decomposition to short-chain dicarboxylic acids or even hydrogen gas," Han said, "Several new ideas have recently been proposed and tested for utilizing lignin oligomers, such as transformation to photocatalyst, antioxidant, or use as a battery binder."
"In this review, our main goal is to provide readers with timely and accurate latest research progress on oligomeric product formation during depolymerization, which is a much-needed topic in the lignin valorization field," Singh said.
Industrial Chemistry & Materials is a peer-reviewed interdisciplinary academic journal published by Royal Society of Chemistry (RSC) with APCs currently waived. Icm publishes significant innovative research and major technological breakthroughs in all aspects of industrial chemistry and materials, especially the important innovation of the low-carbon chemical industry, energy, and functional materials.
END