Why do some animals regenerate lost tissues after injury while others don’t? Researchers from the lab of Kerstin Bartscherer (Osnabrück University and formerly Hubrecht Institute) and Ashley Seifert (University of Kentucky) studied spiny mice, which have a remarkable regenerative capacity, to answer this question. They compared and modulated the injury responses of these mice and common laboratory mice, that show scarring upon injury. This revealed that ERK signalling is a crucial molecular switch between scarring and regeneration. The results of this study will be published on April 26th in the scientific journal Science Advances and imply that modulation of ERK signalling could potentially be used to stimulate regeneration in a clinical setting.
It’s estimated that around 50% of all people die from a disease that involves scarring. Scarring is the result of a limited capacity to regenerate lost and damaged parts of the human body. Permanent scars hinder the normal function of our organs and can therefore lead to organ failure. Strikingly, some animals do not show extensive scarring and are able to regenerate most organs of their body after injury. Lessons learned from these animals hold great promise to stimulate regeneration in humans.
Scarring: not a spiny problem
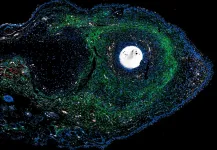
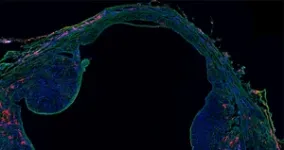
The researchers studied one of these animals: the spiny mouse. Spiny mice are closely related to common laboratory mice. Despite this close relation, spiny mice harbour a remarkable capacity to regenerate complex tissues that common laboratory mice lack. “To study this, we provoke an injury response in the two mouse species by small ear punches. While the spiny mouse repairs the hole in the ear within a month, the hole in the laboratory mouse ear fails to heal,” explains Antonio Tomasso, first author of the study. The researchers then compared the injury responses on a molecular level to identify new factors that could explain regenerative differences between species.
ERK signalling
Doing so, the authors identified the signalling protein ERK as one of the factors that was differently regulated between the mouse species after injury. Tomasso explains: “While ERK was initially activated in the wounds of both species, this activity was rapidly lost in the common laboratory mouse. Spiny mice on the other hand maintained the ERK activation for a longer period”. ERK signalling is known for its important role in processes such as cell division and survival. Now, the authors discovered that the late activation of ERK is also paramount for the regenerative success of the spiny mouse ear, since ERK inhibition prevented successful regeneration of the tissue.
Stimulating regeneration
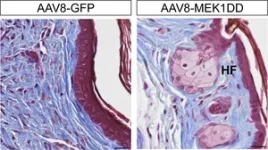
The researchers then investigated if stimulating late ERK activity in the non-regenerating laboratory mouse ear would be sufficient to improve regeneration. For this, they implanted tiny beads into the ear that were soaked in activators of the ERK protein. The beads locally release these factors, which leads to activation of the ERK signalling pathway in the damaged tissue. “We saw that the implantation of the beads led amongst others to controlled tissue growth, cell cycle activation and the formation of hair follicles: all hallmarks of a true regenerative response,” says Tomasso. From this, the researchers concluded that modulation of ERK activity can be used to stimulate regeneration in tissues where this does not naturally occur.
Regeneration in other organs
The results of the study clearly demonstrate that poor regenerators like laboratory mice have not completely lost the capacity to regenerate lost tissues. Instead, it shows that the activation of factors, such as ERK, could have the potential to stimulate regeneration in the context of scarring. Strikingly, the researchers observed that ERK activation was also maintained at late timepoints in the context of back skin and heart injury of spiny mice. “We have not studied the effect of ERK activation on the regeneration of other organs in detail yet. Nevertheless, the fact that the activation pattern is highly similar in other organs indicates that prolonged ERK signalling might be a general feature of regenerating tissues,” says group leader Kerstin Bartscherer. She concludes: “It is therefore possible that the modulation of ERK activation has the potential to prevent or reverse scar formation in other species as well. More research is needed to confirm this”.
*****
Publication
An ERK-dependent molecular switch antagonizes fibrosis and promotes regeneration in spiny mice (Acomys). Antonio Tomasso, Tim Koopmans, Philip Lijnzaad, Kerstin Bartscherer, Ashley W. Seifert. Science Advances, 2023.
*****
Group leader
Kerstin Bartscherer is a former group leader at the Hubrecht Institute. She recently moved to the University of Osnabrück.
*****
About the Hubrecht Institute
The Hubrecht Institute is a research institute focused on developmental and stem cell biology. Because of the dynamic character of the research, the institute as a variable number of research group, around 20, that do fundamental, multidisciplinary research on healthy and diseased cells, tissues and organisms. The Hubrecht Institute is a research institute of the Royal Netherlands Academy of Arts and Sciences (KNAW), situated on Utrecht Science Park. Since 2008, the institute is affiliated with the UMC Utrecht, advancing the translation of research to the clinic. The Hubrecht Institute has a partnership with the European Molecular Biology Laboratory (EMBL). For more information, visit www.hubrecht.eu.
END