The recipients are Gallogly College of Engineering faculty Vivek Bajpai, Ph.D., John R. Clegg, Ph.D., and Stefan Wilhelm, Ph.D. The highly competitive five-year, $1,866,485 grants will support their ambitious research programs without the need to recompete for funding throughout the duration of their awards.
Bajpai, an assistant professor in the School of Sustainable Chemical, Biological and Materials Engineering, will lead the project, “Epigenetic and Transcriptional Mechanisms Driving Human Pigmentation Diversity.”
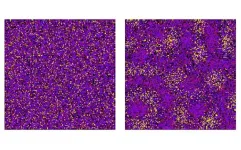
Using the gene-editing technology CRISPR, Bajpai led a recent discovery of 135 new melanin genes associated with human pigmentation.
“We discovered new factors (genes) which are present in our DNA that make us unique in terms of color and creates diversity among the human population,” Bajpai said. “But now the bigger questions are which of these genes are most important? How do changes in these genes cause pigmentation diseases, including melanoma, and can we exploit the power of these genes to engineer therapies against diseases? Our lab is utilizing cutting-edge technologies, diverse model systems and engineering principles to propel this project, which has both fundamental and applied directions.”
For this project, Bajpai’s research group will explore what controls the genes that make up pigment melanin in the skin – currently an open question in the field. He is trying to understand how these genes relate to one another and other genes in the body to determine if there are “critical nodes” that may be important for driving major shifts in the process of melanin synthesis that results in pigmentation.
“We found a class of genes called transcription factors,” he said. “These are the proteins which bind to the DNA, then they affect the production of many other proteins in the cells. In terms of gene functioning, some of the genes could be in a hierarchy and regulating a bunch of other genes, and we are further investigating this.”
Another aspect is the clinical and translational application of his project. Some market estimates cite the global market of pigmentation disorders treatments is $7 billion annually and is projected to reach $9.2 billion by 2028.
“Clearly, there is an unmet need,” Bajpai said. He envisions manipulating these genes’ actions in patients’ skin to obtain the desired pigmentation levels in patients who are suffering from hyper or hypopigmentation diseases.
This award will also allow his research group to investigate the link between lighter skin color and melanoma susceptibility. According to the Centers for Disease Control and Prevention, about 6 million people each year in the United States are treated for skin cancer of any kind, but of those, melanoma causes the most deaths among all types of skin cancer, and incidence rates have increased over time. Findings from a 2016 National Institutes of Health report showed overall melanoma mortality was higher in white non-Hispanics in Oklahoma than the national average.
Many of the 135 melanin genes Bajpai discovered are present at different levels in light- and dark-skinned humans. He believes their variable levels in skin cells contribute toward melanoma initiation. “By having a finer understanding of how these genes work in melanoma and manipulating their levels, we intend to engineer therapeutic strategies,” he said.
Clegg, an assistant professor of biomedical engineering, will lead the project, “Enabled by drug delivery: Studying the role of brain-resident and infiltrating myeloid cell phenotype in brain damage associated with inflammatory disease.”
Inflammation in the brain can have many causes, including traumatic injury, stroke, infection, some drug treatments or exposure to environmental toxins. When inflammation persists over weeks, months or even patients’ lifespans, persistent neurological disorders can result.
“Chronic inflammation underlies cognitive decline and neurodegenerative diseases,” Clegg said. “What’s wild is that there are no approved therapeutics to intervene in this inflammatory aspect of neurological diseases.”
While therapeutics are available to help patients manage symptoms or to help with functional issues such as memory, and physical therapy is available to help patients recover motor function, there are no approved therapeutics to address inflammation specifically.
“So far, clinical trials on anti-inflammatory therapy for brain injury have been unsuccessful,” he added. “This has motivated us to study the different immune cell populations that contribute to brain inflammation and to develop drug delivery systems that reduce brain inflammation in a targeted manner.”
Clegg’s research aims to fill the gap in scientists’ understanding of how to use drug delivery systems to intervene following various forms of brain injury.
Broadly, his research examines the efficacy of immunomodulatory therapeutics – a class of medical treatments that aim to modify the immune system – when delivered either locally to immune cells within the brain or globally to immune cells that are in other tissues or circulating in the body.
Clegg’s research group is developing new and creative ways to deliver immune-modulating compounds using nanomaterials and hydrogels. These delivery systems can be designed to target immune cells that cause or exacerbate brain inflammation.
“My lab’s projects generally fall into one of three areas, and this award focuses on two of those overarching topics,” Clegg said. “We are making new nanomaterials that target myeloid cells, with emphasis on bone marrow-derived immune cells. Second, we’re developing injectable gels that form anti-inflammatory niches for local drug delivery in the brain.”
The third area, outside of the scope of this award, is fabricating new model systems to test drug delivery materials.
Wilhelm, an associate professor of biomedical engineering, leads the project, “A novel framework for nanomedicine development.”
Building on the research that contributed to the development of mRNA vaccines for COVID-19, Wilhelm is using this award to develop a new generation of lipid nanoparticles to potentially treat a wide range of diseases.
“We are proposing various types of surface modifications for lipid nanoparticles that involve different types of synthetic polymers and biomolecules,” Wilhelm said. “Our hope is that we can tailor the targeting specificity of these nanoparticles to various cell types, opening up new possibilities where these types of nanomaterials can be used for diseases other than infectious diseases.”
However, Wilhelm cautions that a challenge with lipid nanoparticle technology is that the safety and efficacy depend on controlling where those nanoparticles end up in the body.
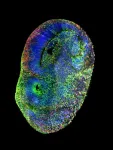
Wilhelm’s lab has applied a 3D super-resolution imaging technique, known as expansion microscopy, combined with a technique for imaging metallic nanoparticles within cells to realize a significantly improved ability to see nanoparticles within cells.
“We have shown in the literature now that we can do super-resolution imaging of cells containing metallic nanoparticles. Now, we are switching gears, and we are essentially translating what we have developed so far with metallic nanoparticles to also include lipid nanoparticles,” he said. “With our experience in imaging technologies and our expertise in nanomaterial synthesis and characterization, we are now putting these together to create a new generation of safer, more effective, and more efficient lipid nanoparticles that can be used to deliver payloads to target cells in the body against different types of diseases.”
This proof-of-concept research aims to show that by understanding the transport and characteristics like the biodistribution of these nanoparticles inside cells, customized therapeutics can be developed that are safe and effective.
###
About the University of Oklahoma
Founded in 1890, the University of Oklahoma is a public research university located in Norman, Oklahoma. OU serves the educational, cultural, economic and health care needs of the state, region and nation. For more information visit www.ou.edu.
END