Lithium-ion batteries (LIBs) are, by far, the most widely used type of rechargeable batteries, spanning numerous applications. These include consumer electronics, electric vehicles (e.g., Tesla cars), renewable energy systems, and spacecrafts. Although LIBs deliver the best performance in many aspects when compared to other rechargeable batteries, they have their fair share of disadvantages. Lithium is a rather scarce resource, and its price will rise quickly with its availability going down in the future. Moreover, lithium extraction and improperly discarded LIBs pose huge environmental challenges as the liquid electrolytes commonly used in them are toxic and flammable.
The shortcomings of LIBs have motivated researchers worldwide to look for alternative energy storage technologies. Sodium (Na)-ion batteries (NIBs) and potassium-ion batteries (KIBs) are two rapidly emerging options that are cost-efficient as well as sustainable. Both NIBs and KIBs are projected to be billion-dollar industries by the end of the decade. Governments across the world, including that of the US, Austria, Hong Kong, Germany, and Australia, are promoting research and innovation in this field. Moreover, companies such as Faradion Limited, TIAMAT SAS, and HiNa Battery Technology Co. Ltd., are investing heavily in this technology. Both Contemporary Amperex Technology Co. Limited and Build Your Dreams are expected to introduce electric vehicle battery packs with NIBs soon.
Unfortunately, however, the capacity of the electrode materials used in NIBs and KIBs still lags behind that of LIBs. Against this backdrop, a research team led by Professor Shinichi Komaba from Tokyo University Science (TUS), Japan, has been working to develop groundbreaking high-capacity electrode materials for NIBs and KIBs. In their latest study, published in Advanced Energy Materials on November 13, 2023, they report a new synthesis strategy for nanostructured “hard carbon” (HC) electrodes that deliver unprecedented performance. The study was co-authored by Mr. Daisuke Igarashi, Ms. Yoko Tanaka, and Junior Associate Professor Ryoichi Tatara from TUS, and Dr. Kei Kubota from the National Institute for Materials Science (NIMS), Japan.
But what is HC and why is it useful for NIBs and KIBs? Unlike other forms of carbon, such as graphene or diamond, HC is amorphous; it lacks a well-defined crystalline structure. Additionally, it is strong and resistant. In an earlier 2021 study, Prof. Komaba and his colleagues had found a way to use magnesium oxide (MgO) as a template during the synthesis of HC electrodes for NIBs, altering their final nanostructure. The process had led to the formation of nanopores within the electrodes upon MgO removal, which, in turn, had vastly increased their capacity to store Na+ ions.
Motivated by their previous findings, the researchers explored whether compounds made from zinc (Zn) and calcium (Ca) could also be useful as nano-templates for HC electrodes. To this end, they systematically investigated different HC samples made using zinc oxide (ZnO) and calcium carbonate (CaCO3) and compared their performance with the ones synthesized using magnesium oxide (MgO).
Preliminary experiments showed that ZnO was particularly promising for the negative electrode of NIBs. Accordingly, the researchers optimized the concentration of ZnO embedded in the HC matrix during synthesis, demonstrating a reversible capacity of 464 mAh g–1 (corresponding to NaC4.8) with a high initial Coulombic efficiency of 91.7% and a low average potential of 0.18 V vs. Na+/Na.
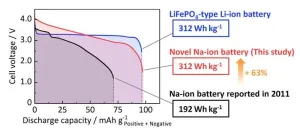
The team achieved remarkable results by incorporating this powerful electrode material into an actual battery. “The NIB fabricated using the optimized ZnO-templated HC as the negative electrode exhibited an energy density of 312 Wh kg–1,” highlights Prof. Komaba. “This value is equivalent to the energy density of certain types of currently commercialized LIBs with LiFePO4 and graphite and is more than 1.6 times the energy density of the first NIBs (192 Wh kg–1), which our laboratory reported back in 2011.” Notably, the ZnO-templated HC also exhibited a significant capacity of 381 mAh g–1 when incorporated into a KIB, further showcasing its potential.
Taken together, the results of this study show that using inorganic nanoparticles as a template to control the pore structure may provide an effective guideline for the development of HC electrodes. “Our findings prove that HCs are promising candidates for negative electrodes as an alternative to graphite,” concludes Prof. Komaba.
In turn, this could make NIBs viable for practical applications, such as the development of sustainable consumer electronics and electric vehicles as well as low carbon footprint energy storage systems for storing energy from solar and wind farms.
***
Reference
DOI: https://doi.org/10.1002/aenm.202302647
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society,” TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Professor Shinichi Komaba from Tokyo University of Science
Prof. Shinichi Komaba obtained his Ph.D. from Waseda University, Japan, and then joined Iwate University as a research associate. After studying as a post-doctoral researcher at Bordeaux-CNRS, France, he joined Tokyo University of Science in 2005 to work on developing electrodes, electrolytes, and binding materials for various types of rechargeable batteries. His research group carries out cutting-edge research in the field of rechargeable batteries and their electrochemical applications. With more than 290 publications to his credit, Prof. Komaba has won numerous international awards, including the title of "Highly Cited Researcher" from 2019 to 2022.
Laboratory website
Official TUS website
END