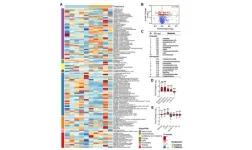
(MEMPHIS, Tenn. – April 9, 2024) Scientists from St. Jude Children’s Research Hospital today announced the St. Jude survivorship portal — the first data portal for sharing, analyzing and visualizing pediatric cancer survivorship data. Details on the portal and its ability to facilitate breakthroughs in pediatric cancer survivorship research were published today in Cancer Discovery, a journal of the American Association for Cancer Research.
The survivorship portal is a big-data platform that incorporates clinical and genomic information, creating an unprecedented research system. The portal integrates three dimensions of data: whole genomic sequencing, treatment exposure and outcomes. It houses 1,600 phenotypic variables and 400 million genetic variants from over 7,700 childhood cancer survivors. The portal is free to use and open access as a part of the St. Jude Cloud ecosystem.
“With the portal, with just one click, you can make new discoveries,” said co-corresponding author Jinghui Zhang, PhD, St. Jude Department of Computational Biology. “In the past, people would spend weeks downloading, analyzing, organizing and summarizing data into figures — now you can do all that in just minutes.”
Variable summary statistics are computed dynamically and visualized through interactive and customizable charts. Users can assemble custom cohorts by specifying clinical and genetic variables for comparative analysis. The portal provides a range of additional features, including genome exploration, statistical testing and cumulative incidence and regression analysis, with open access and free computing.
“Continued enhancement on the portal architecture is key to enable on-the-fly analysis, which integrates data on treatment exposures, whole genome sequencing, and long-term outcomes,” said co-corresponding author Xin Zhou, PhD, St. Jude Department of Computational Biology.
St. Jude survivorship cohorts provide rich data
Approximately 85% of childhood cancer patients are successfully treated and alive five years post-diagnosis, with most going on to live much longer. This growing population of survivors is at risk of developing a wide range of adverse health effects that can be linked back to their cancer or its treatment. Such outcomes include premature mortality, organ dysfunction, secondary cancers and psychosocial challenges, among others. By studying this unique population of childhood cancer survivors, investigators can gain insight into how to best tailor therapy up-front and provide screening and support later in life to avoid adverse outcomes.
St. Jude is well positioned to contribute to survivorship research because it is home to two significant survivorship cohorts: the Childhood Cancer Survivor Study (CCSS) and the St. Jude Lifetime Cohort (St. Jude LIFE). CCSS is a collaborative effort representing 31 institutions spanning North America and compiling data on a range of childhood cancers. St. Jude LIFE is a long-term follow-up study where St. Jude patients are brought back to the hospital every five years for an assessment. Together, these cohorts provide a rich data source for investigators to mine for new insights.
“There are half a billion clinical data points in the portal, hundreds of terabytes of genetic data supported by dynamic and interactive visualization analysis,” said Zhou.
“We aren’t just sharing data,” added Yutaka Yasui, PhD, St. Jude Department of Epidemiology and Cancer Control. “We are facilitating the analysis and visualization of data and making it free to anyone — that’s a tremendous resource for the cancer survivorship community.”
Portal leads to new findings
In the Cancer Discovery report, the researchers share examples of findings they made using the portal. For example, platinum chemotherapy has been used for decades to treat many types of cancer; however, these agents are known to cause auditory (hearing) toxicity. The researchers compared the use and associated outcomes for two types of platinum chemotherapy: cisplatin and carboplatin. Their findings showed cisplatin was associated with greater auditory toxicity than carboplatin.
“By enabling the study of the mechanisms underlying toxicity, the portal can really inform investigators how to prioritize drugs for treatment,” Zhang said. “Investigators can come to the portal with different interests, genetics, drug usage and exposure or survivorship — these perspectives can all be explored in the portal.”
Additionally, the researchers illustrated the portal’s utility by discovering a novel association between mental health, age and limb amputation: Receiving an amputation at an older age (teenage compared to earlier childhood) is associated with increased resilience against poor mental health.
The researchers also demonstrated the portal’s ability to explore data related to individuals of specific genetic ancestry. Previous studies reported a higher risk of cardiomyopathy (heart disease) among childhood cancer survivors of African ancestry. Using the portal to explore the genetic basis underlying increased risk, the researchers discovered a novel set of DNA variants in MAGI3, which was strongly associated with cardiomyopathy risk in survivors of African ancestry.
These finding exemplify the breadth and depth of discoveries that can be made through the St. Jude survivorship portal.
“An early-career or scientist without a computing background can come to the portal with a question in mind and, with just a few minutes and a few clicks, become a kind of bioinformatics scientist or epidemiologist,” Zhang said.
Authors and funding
The study’s first authors are Gavriel Matt, Edgar Sioson, Kyla Shelton and Jian Wang of St. Jude. Additional authors are Congyu Lu, Airen Zaldivar Peraza, Karishma Gangwani, Robin Paul, Colleen Reilly, Aleksandar Acic, Qi Liu, Stephanie Sandor, Clay McLeod, Jaimin Patel, Fan Wang, Cindy Im, Zhaoming Wang, Yadav Sapkota, Carmen Wilson, Nickhill Bhakta, Kirsten Ness, Gregory Armstrong, Melissa Hudson and Leslie Robison, all of St. Jude.
The study was supported by grants from the National Institutes of Health (U01CA195547, R01CA216354, R01CA270157, U24CA55727, R01CA261898 and CA21765) and ALSAC, the fundraising and awareness organization of St. Jude.
St. Jude Media Relations Contacts
Gary Bridgman
Desk: (901) 595-2028
gary.bridgman@stjude.org
media@stjude.org
Chelsea Bryant
Cell: (256) 244-2048
Desk: (901) 595-0564
chelsea.bryant@stjude.org
media@stjude.org
Rae Lyn Hartley
Cell: (901) 686-2597
raelyn.hartley@stjude.org
media@stjude.org
St. Jude Children's Research Hospital
St. Jude Children's Research Hospital is leading the way the world understands, treats and cures childhood cancer, sickle cell disease and other life-threatening disorders. It is the only National Cancer Institute-designated Comprehensive Cancer Center devoted solely to children. Treatments developed at St. Jude have helped push the overall childhood cancer survival rate from 20% to 80% since the hospital opened more than 60 years ago. St. Jude shares the breakthroughs it makes to help doctors and researchers at local hospitals and cancer centers around the world improve the quality of treatment and care for even more children. To learn more, visit stjude.org, read St. Jude Progress, a digital magazine, and follow St. Jude on social media at @stjuderesearch.
END