(Press-News.org) Lisbon, Portugal – 13 May 2024: Even though mortality and hospitalisation rates have improved, the quality of life for those living with hypertrophic cardiomyopathy (HCM) can be compromised with limiting symptoms such as exertional dyspnoea and decreased exercise capacity. A major cause of this in HCM patients is left ventricular outflow tract (LVOT) obstruction, which results in elevated intracardiac pressures. This study demonstrated that aficamten enhanced HCM patients’ exercise capacity with significant improvement in peak oxygen uptake (pVO2), improvement in limiting symptoms, and decreases in LVOT pressure gradients. The late breaking research presented today at Heart Failure 2024, a scientific congress of the European Society of Cardiology (ESC).1
“The SEQUOIA-HCM trial demonstrated that aficamten can reliably and safely eliminate LVOT obstruction in patients with obstructive HCM using a simple and stepwise dosing regimen, and was associated with substantial improvements in clinically relevant endpoints such as exercise capacity and symptoms,” said principal investigator Professor Martin Maron of the Lahey Hospital and Medical Center, Burlington, Massachusetts, US. “HCM patients are often on multiple medications, which frequently provide suboptimal benefit, while aficamten was highly effective at providing clinical improvement as combination therapy, but also as monotherapy.”
HCM occurs in approximately one in 200 to 500 individuals, with 70% of patients having obstructive disease.2 The condition causes the walls of the left ventricle to become thick and stiff, which can also result in obstruction to blood flow out of the heart and increased intracardiac pressures.
Aficamten is a cardiac myosin inhibitor that was previously shown to reduce LVOT gradients in a phase 2 trial.3 The phase 3 SEQUOIA-HCM trial evaluated the efficacy and safety of aficamten versus placebo in adults with symptomatic obstructive HCM. The primary endpoint was the change in pVO2, assessed using cardiopulmonary exercise testing, from baseline to week 24. Secondary endpoints at 24 weeks included the change in KCCQ score; the proportion of patients with ≥1 class improvement in New York Heart Association (NYHA); change in Valsalva LVOT gradient; the proportion of patients with Valsalva LVOT gradient <30 mmHg; and eligibility for invasive septal reduction.
SEQUOIA-HCM included 282 patients from 101 sites in 14 countries in North America, Asia, and Europe, making it the largest-ever obstructive HCM trial. All participants had reduced exercise capacity due to obstructive HCM. Patients were randomised 1:1 to aficamten or placebo on top of their background medical therapy. The starting dose of aficamten was 5 mg once daily with opportunities at weeks 2, 4, and 6 to increase the dose in 5 mg increments to a maximum dose of 20 mg. Dose adjustments were made according to left ventricular ejection fraction and LVOT gradients assessed using echocardiography.
The mean increase in pVO2 from baseline to 24 weeks was 1.8 ml/kg/min with aficamten compared to 0.0 ml/kg/min with placebo (least-squares mean difference between groups, 1.7 ml/kg/min; 95% confidence interval [CI] 1.0, 2.4; p<0.001). Regarding secondary endpoints at 24 weeks, aficamten resulted in a least-squares mean difference of 7 points in KCCQ score relative to placebo (95% CI 5, 10; p<0.0001). A ≥1 NYHA class improvement was observed in 58.5% of patients on aficamten and 24.3% of patients on placebo (p<0.0001). Aficamten led to a 50 mmHg greater reduction in Valsalva LVOT gradient versus placebo (95% CI -57, -44; p<0.0001). Some 49.3% of patients on aficamten achieved a Valsalva LVOT gradient <30 mmHg versus 3.6% of patients on placebo (p<0.0001). The aficamten group had 78 fewer days eligible for invasive septal reduction compared with the placebo group (p<0.0001).
Professor Maron said: “It was impressive to see that the beneficial effects of aficamten occurred rapidly and consistently over the treatment period and that the doses could be adjusted effectively and safely using only site read echocardiographic measures. It was also reassuring to see that in the very small number of patients found to have an ejection fraction below 50% on aficamten, there was no associated heart failure or the need for dose interruption, and that the effect on ejection fraction was reversible with treatment discontinuation.”
Authors: ESC Press Office
Tel: +33 (0)489 872 075
Email: press@escardio.org
Follow us on X @ESCardioNews
Notes to editor
Funding: Cytokinetics Inc. provided the funding for this study
Disclosures: Martin S. Maron has received Consultant/Advisor fees from Imbria, Edgewise, Biomarin and Steering Committee fees for SEQUOIA-HCM from Cytokinetics, Incorporated. Ahmad Masri has received Consultant/Advisor fees from Tenaya, Attralus, Cytokinetics, Bristol Myers Squibb, and Ionis, and Research Grants from Ionis, Akcea, Pfizer, Ultromics, and Wheeler Foundation. Michael E. Nassif has received Research and grant support from Astra Zeneca and Cytokinetics, Consultant/Advisor fees from Vifor, and Cytokinetics. Roberto Barriales-Villa has received Consultant/Advisor fees from MyoKardia/Bristol Myers Squibb. Michael Arad reports consultant and lecture fees from Bristol Myers Squibb. Nuno Cardim has received Consultant/Advisor fees from Cytokinetics, Bristol Myers Squibb, Pfizer, Menarini, Boehinger-Ingelheim, Bial. Caroline J. Coats has received speaker fees from Alnylam and Roche, and advisory fees from Cytokinetics. Hans-Dirk Düngen reports grants from Novartis, CSL Behring, and Cytokinetics. Pablo Garcia-Pavia has received Speakers’ Bureau fees from Pfizer, AstraZeneca, Novo Nordisk, Ionis, Bridgebio, BMS, Intelllia and Alnylam, Consultant/Advisor fees from Pfizer, Alnylam, MyoKardia/Bristol Myers Squibb, Cytokinetics, Neuroimmune, Intellia, Ionis, Bridgebio, Lexeo, Rocket, Attralus, and AstraZeneca, Research/Educational Grants to his institution from Pfizer, AstraZeneca, Novo Nordisk, BridgeBio, and Alnylam. Albert A. Hagège has received Consultant/Advisor fees from Alnylam, Amicus Therapeutics, Bayer, MyoKardia/Bristol Myers Squibb, Pfizer, Sanofi Genzyme, and Steering Committee fees for SEQUOIA-HCM from Cytokinetics, Incorporated. James L. Januzzi is funded in part by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; has served as a board member for Imbria Pharmaceuticals; has served as a Director at Jana Care; has received grant support from Abbott, Applied Therapeutics, HeartFlow, Innolife, and Roche Diagnostics; has received consulting income from Abbott, Beckman, Bristol Myers Squibb, Boehringer Ingelheim, Janssen, Novartis, Pfizer, Merck, Roche Diagnostics, and Siemens; and has participated in clinical end point committees/data safety monitoring boards for Abbott, AbbVie, CVRx, Intercept, and Takeda. Matthew M.Y. Lee has received Research Grants through his institution from AstraZeneca, Boehringer Ingelheim, and Roche Diagnostics, and is a member of a Clinical Endpoints Committee for Bayer, and a Trial Steering Committee for Cytokinetics. Gregory D. Lewis has received research funding from the National Institutes of Health (R01-HL 151841, R01-HL131029, and R01-HL159514), American Heart Association (15GPSGC-24800006), Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, and SoniVie, honoraria for advisory boards from Pfizer, Merck, Boehringer Ingelheim, Novartis, American Regent, Cyclerion, Cytokinetics, and Amgen, and royalties from UpToDate for scientific content authorship related to exercise physiology. Michelle Michels has received Consultant/ Advisor fees from Bristol Myers Squibb, Cytokinetics, Pfizer and Research Grant funding from Bristol Myers Squibb. Iacopo Olivotto has received Speakers’ Bureau fees from Bristol Myers Squibb, Amicus, Sanofi Genzyme, Consultant/Advisor fees from Bristol Myers Squibb, Cytokinetics, Sanofi Genzyme, Amicus, Bayer, Tenaya, Rocket Pharma and Lexeo, and Research Grant funding from Bristol Myers Squibb, Cytokinetics, Sanofi Genzyme, Amicus, Bayer, Menarini International, Chiesi and Boston Scientific. Artur Oreziak has received investigator fees from Cytokinetics and MyoKardia/Bristol Myers Squibb. Anjali T. Owens has received Consultant/Advisor fees from Cytokinetics, Bristol Myers Squibb/MyoKardia, and Pfizer. John A. Spertus is the PI of grants from the NIH, Abbott Vascular, and the American College of Cardiology Foundation; is a consultant for Janssen, Novartis, Amgen, Myokardia, AstraZeneca, Bayer, and Merck; serves on the Scientific Advisory Board of United Healthcare and the Board of Directors for Blue Cross Blue Shield of Kansas City; owns the copyright to the KCCQ, SAQ, and PAQ; and has an equity interest in Health Outcomes Sciences. Scott D. Solomon has received Consultant/Advisor fees from Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GSK, Lilly, Merck, MyoKardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, and Puretech Health, and Research Grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI. Jacob Tfelt-Hansen is a consultant for Leo Pharma, MicroPort, and Johnson and Johnson. Marion van Sinttruije is a patient advisory committee member and a SEQUOIA-HCM Steering Committee member for Cytokinetics Incorporated. Hugh C. Watkins has received Consultant/Advisor fees from Cytokinetics, BioMarin, and BridgeBio. Daniel L. Jacoby, Stephen B. Heitner, Stuart Kupfer, Fady I. Malik, Lisa Meng, and Amy Wohltman are employees of Cytokinetics Incorporated and hold stock in Cytokinetics Incorporated. Lubna Choudhury, Brian Claggett, Chang-Sheng Ma, Josef Veselka, and Theodore P. Abraham have no disclosures to report.
References and notes
1The ‘SEQUOIA-HCM’ trial will be presented during the session ‘Late Breaking Clinical trials: LVAD, HFpEF and hypertrophic cardiomyopathy’ which takes place on 13 May 2024 in Room 1.
2Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239.
3Maron MS, Masri A, Choudhury L, et al. Phase 2 study of aficamten in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2023;81:34–45.
About Heart Failure 2024 & World Congress on Acute Heart Failure
Heart Failure is the annual congress of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). #HeartFailure2024
About the Heart Failure Association
The Heart Failure Association (HFA) is a branch of the European Society of Cardiology (ESC). Its aim is to improve quality of life and longevity, through better prevention, diagnosis and treatment of heart failure, including the establishment of networks for its management, education and research.
About the European Society of Cardiology
The European Society of Cardiology brings together health care professionals from more than 150 countries, working to advance cardiovascular medicine and help people lead longer, healthier lives.
Information for journalists about registration for Heart Failure 2024
Heart Failure 2024 takes place 11 to 14 May at the Lisbon Congress Centre, Portugal and online. Explore the scientific programme.
Free registration applies to accredited press.
Credentials: A valid press card or appropriate letter of assignment with proof of three recent published articles. Read the ESC media and embargo policy.
The ESC Press Office will verify the documents and confirm by email that your press accreditation is valid.
The ESC Press Office decision is final regarding all press registration requests. END
SEQUOIA-HCM trial meets primary endpoint in obstructive hypertrophic cardiomyopathy
SEQUOIA-HCM trial presented in a late breaking science session today at Heart Failure 2024
2024-05-13
ELSE PRESS RELEASES FROM THIS DATE:
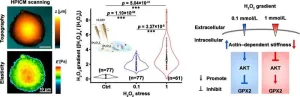
Exploring the effect of H2O2 eustress on individual cancer cells using hopping probe scanning ion conductance microscopy (HPICM)
2024-05-13
In a recent study published in the multidisciplinary academic journal Science Bulletin, a semi-monthly high-caliber peer-reviewed research outlet covering a broad range of natural sciences and high-tech fields, researchers from the Nano Life Science Institute (WPI-NanoLSI) at Kanazawa University utilized hopping probe scanning ion conductance microscopy (HPICM) and highly sensitive platinum-functionalized nanoelectrodes to conduct an in-depth investigation of the dynamic response of individual living colorectal cancer Caco-2 cells to changes ...
European Society of Endocrinology and Endocrine Society publish Joint Guideline on glucocorticoid-induced adrenal insufficiency (GC-AI)
2024-05-13
European Society of Endocrinology and Endocrine Society publish Joint Guideline on Glucocorticoid-induced Adrenal Insufficiency (GC-AI)
As focal points for endocrinology and hormone research, both the European Society of Endocrinology (ESE) and the Endocrine Society (ES) regularly produce clinical guidelines with recommendations for patient care, either in collaboration with other Societies or independently. Guideline development at each society is overseen by a Clinical Committee, and all guidelines are subject to a rigorous review process before ...
Endocrine Society and European Society of Endocrinology publish joint guideline on glucocorticoid-induced adrenal insufficiency
2024-05-13
WASHINGTON—The joint guideline is designed to help clinicians manage patients who have, or are at risk of developing, glucocorticoid-induced adrenal insufficiency. At least 1% of the global population uses chronic glucocorticoid therapy as anti-inflammatory or immune-suppressive agents.
The guideline, titled “Diagnosis and Therapy of Glucocorticoid-induced Adrenal Insufficiency,” will appear in the May 2024 issues of the Societies’ respective journals, The Journal of Clinical Endocrinology & Metabolism and the European Journal of Endocrinology. ...
Some varieties of annual flowers have a place in pollinator-friendly gardens
2024-05-13
Annapolis, MD; May 13, 2024—While wildflowers and perennials are a must for supporting pollinators, there's no denying the popularity of many annual flowers for their colorful, visual appeal. Annuals are often thought of as pollinator "deserts," but a new study suggests choosing the right varieties can give annual flowers a role in nourishing bees and other pollinating insects in home gardens.
In a two-year study, researchers at Michigan State University observed pollinators visiting 25 different varieties, or cultivars, of the six most popular annual ...
Plant virus treatment shows promise in fighting metastatic cancers in mice
2024-05-13
An experimental treatment made from a plant virus is effective at protecting against a broad range of metastatic cancers in mice, shows a new study from the University of California San Diego.
The treatment, composed of nanoparticles fashioned from the cowpea mosaic virus—a virus that infects black-eyed pea plants—showed remarkable success in improving survival rates and suppressing the growth of metastatic tumors across various cancer models, including colon, ovarian, melanoma and breast cancer. Similar outcomes were also observed when the treatment was administered to mice whose tumors were surgically removed.
The findings were published recently in Advanced Science.
The ...
SwRI studies boiling processes in partial gravity aboard parabolic flights
2024-05-13
SAN ANTONIO — May 13, 2024 —Southwest Research Institute is studying the process of boiling liquids under partial gravity conditions in a series of parabolic flights. The internally funded project, conducted in collaboration with Texas A&M University, aims to better understand how liquids boil on different surfaces in partial gravity. Boiling liquids will likely be required during future extended space missions to the Moon or Mars to support surface power, life support systems, cryogenic fuel production and in situ resource utilization.
“We ...
Prostate cancer study: More health benefits from plant-based diet
2024-05-13
Men with prostate cancer could significantly reduce the chances of the disease worsening by eating more fruits, vegetables, nuts, and olive oil, according to new research by UC San Francisco.
A study of more than 2,000 men with localized prostate cancer found that eating a primarily plant-based diet was associated with a 47% lower risk that their cancer would progress, compared with those who consumed the most animal products.
This amounted to eating just one or two more servings per day of healthy foods, particularly vegetables, fruits, and whole grains, while eating fewer animal products, like dairy and meat. The study followed ...
When consumers would prefer a chatbot over a person
2024-05-13
COLUMBUS, Ohio – Actually, sometimes consumers don’t want to talk to a real person when they’re shopping online, a new study suggests.
In fact, what they really want is a chatbot that makes it clear that it is not human at all.
In a new study, researchers at The Ohio State University found that people preferred interacting with chatbots when they felt embarrassed about what they were buying online – items like antidiarrheal medicine or, for some people, skin care products.
“In general, research shows people would rather interact with a human customer ...
Intense ultrasound extracts genetic info for less invasive cancer biopsies #ASA186
2024-05-13
OTTAWA, Ontario, May 13, 2024 – Ultrasound imaging offers a valuable and noninvasive way to find and monitor cancerous tumors. However, much of the most crucial information about a cancer, such as specific cell types and mutations, cannot be learned from imaging and requires invasive and damaging biopsies. One research group developed a way to employ ultrasound to extract this genetic information in a gentler way.
At the University of Alberta, a team led by Roger Zemp explored how intense ultrasound can release biological indicators of disease, or biomarkers, from cells. These biomarkers, ...
Weight loss drug linked with reduced need for diuretics in heart failure patients
2024-05-13
Lisbon, Portugal – 13 May 2024: Semaglutide reduces the need for loop diuretic use and dose, and has positive effects on symptoms, physical limitations, and body weight in patients with heart failure with preserved ejection fraction (HFpEF) regardless of diuretic use, according to late breaking research presented today at Heart Failure 2024, a scientific congress of the European Society of Cardiology (ESC).1
HFpEF is a condition in which the heart pumps normally but is too stiff to fill properly, rendering the heart unable to support the body’s need for oxygen-rich blood. The condition is becoming more common as populations ...
LAST 30 PRESS RELEASES:
Scientists discover why we know when to stop scratching an itch
A hidden reason inner ear cells die – and what it means for preventing hearing loss
Researchers discover how tuberculosis bacteria use a “stealth” mechanism to evade the immune system
New microscopy technique lets scientists see cells in unprecedented detail and color
Sometimes less is more: Scientists rethink how to pack medicine into tiny delivery capsules
Scientists build low-cost microscope to study living cells in zero gravity
The Biophysical Journal names Denis V. Titov the 2025 Paper of the Year-Early Career Investigator awardee
Scientists show how your body senses cold—and why menthol feels cool
Scientists deliver new molecule for getting DNA into cells
Study reveals insights about brain regions linked to OCD, informing potential treatments
Does ocean saltiness influence El Niño?
2026 Young Investigators: ONR celebrates new talent tackling warfighter challenges
Genetics help explain who gets the ‘telltale tingle’ from music, art and literature
Many Americans misunderstand medical aid in dying laws
Researchers publish landmark infectious disease study in ‘Science’
New NSF award supports innovative role-playing game approach to strengthening research security in academia
Kumar named to ACMA Emerging Leaders Program for 2026
AI language models could transform aquatic environmental risk assessment
New isotope tools reveal hidden pathways reshaping the global nitrogen cycle
Study reveals how antibiotic structure controls removal from water using biochar
Why chronic pain lasts longer in women: Immune cells offer clues
Toxic exposure creates epigenetic disease risk over 20 generations
More time spent on social media linked to steroid use intentions among boys and men
New study suggests a “kick it while it’s down” approach to cancer treatment could improve cure rates
Milken Institute, Ann Theodore Foundation launch new grant to support clinical trial for potential sarcoidosis treatment
New strategies boost effectiveness of CAR-NK therapy against cancer
Study: Adolescent cannabis use linked to doubling risk of psychotic and bipolar disorders
Invisible harms: drug-related deaths spike after hurricanes and tropical storms
Adolescent cannabis use and risk of psychotic, bipolar, depressive, and anxiety disorders
Anxiety, depression, and care barriers in adults with intellectual and developmental disabilities
[Press-News.org] SEQUOIA-HCM trial meets primary endpoint in obstructive hypertrophic cardiomyopathySEQUOIA-HCM trial presented in a late breaking science session today at Heart Failure 2024