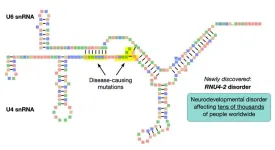
The findings will improve clinical diagnostic services for patients with neurodevelopmental disorders.
Through rigorous genetic analysis, the researchers discovered that mutations in a small non-coding gene called RNU4-2 cause a collection of developmental symptoms that had not previously been tied to a distinct genetic disorder. Non-coding genes are parts of DNA that do not produce proteins. The investigators used whole-genome sequencing data in the United Kingdom's National Genomic Research Library to compare the burden of rare genetic variants in 41,132 non-coding genes between 5,529 unrelated cases with intellectual disability and 46,401 unrelated controls.
The discovery is significant, as it represents one of the most common single-gene genetic causes of such disorders, ranking second only to Rett syndrome among patients sequenced by the United Kingdom's Genomic Medicine Service. Notably, these mutations are typically spontaneous and not inherited, providing important insights into the nature of the condition.
“We performed a large genetic association analysis to identify rare variants in non-coding genes that might be responsible for neurodevelopmental disorders,” says the study's first author Daniel Greene, PhD, Assistant Professor of Genetics and Genomics Sciences at Icahn Mount Sinai and a Visitor at the University of Cambridge. “Nowadays, finding a single gene that harbors genetic variants responsible for tens of thousands of patients with a rare disease is exceptionally unusual. Our discovery eluded researchers for years due to various sequencing and analytical challenges.”
More than 99 percent of genes known to harbor mutations that cause neurodevelopmental disorders encode proteins. The researchers hypothesized that non-coding genes, which don't produce proteins, could also host mutations leading to intellectual disability. Neurodevelopmental disorders, which often appear before grade school, involve developmental deficits affecting personal, social, academic, or occupational functioning. Intellectual disability specifically includes significant limitations in intellectual functioning (e.g., learning, reasoning, problem-solving) and adaptive behavior (e.g., social and practical skills).
“The genetic changes we found affect a very short gene, only 141 units long, but this gene plays a crucial role in a basic biological function of cells, called gene splicing, which is present in all animals, plants and fungi," says senior study author Ernest Turro, PhD, Associate Professor of Genetics and Genomic Sciences at Icahn Mount Sinai and a Visitor at the University of Cambridge. “Most people with a neurodevelopmental disorder do not receive a molecular diagnosis following genetic testing. Thanks to this study, tens of thousands of families will now be able to obtain a molecular diagnosis for their affected family members, bringing many diagnostic odysseys to a close.”
Next, the researchers plan to explore the molecular mechanisms underlying this syndrome experimentally. This deeper understanding aims to provide biological insights that could one day lead to targeted interventions.
"What I found remarkable is how such a common cause of a neurodevelopmental disorder has been missed in the field because we've been focusing on coding genes,” says Heather Mefford, MD, PhD, of the Center for Pediatric Neurological Disease Research at St. Jude Children’s Research Hospital who was not involved with the research. “This study's discovery of mutations in non-coding genes, especially RNU4-2, highlights a significant and previously overlooked cause. It underscores the need to look beyond coding regions, which could reveal many other genetic causes, opening new diagnostic possibilities and research opportunities."
The paper is titled “Mutations in the U4 snRNA gene RNU4-2 cause one of the most prevalent monogenic neurodevelopmental disorders.”
The remaining authors of the paper are Chantal Thys (KU Leuven, Belgium); Ian R. Berry, MD (University of Bristol, UK); Joanna Jarvis, MD (Birmingham Womens’ Hospital, UK); Els Ortibus, MD, PhD (KU Leuven, Belgium); Andrew D. Mumford, MD (University of Bristol, UK); and Kathleen Freson, PhD (KU Leuven, Belgium).
The work was supported, in part, by NIH awards R01HL161365 and R03HD111492. See the paper for further details on funding.
-####-
About the Icahn School of Medicine at Mount Sinai
The Icahn School of Medicine at Mount Sinai is internationally renowned for its outstanding research, educational, and clinical care programs. It is the sole academic partner for the eight- member hospitals* of the Mount Sinai Health System, one of the largest academic health systems in the United States, providing care to a large and diverse patient population.
Ranked 13th nationwide in National Institutes of Health (NIH) funding and among the 99th percentile in research dollars per investigator according to the Association of American Medical Colleges, Icahn Mount Sinai has a talented, productive, and successful faculty. More than 3,000 full-time scientists, educators, and clinicians work within and across 44 academic departments and 36 multidisciplinary institutes, a structure that facilitates tremendous collaboration and synergy. Our emphasis on translational research and therapeutics is evident in such diverse areas as genomics/big data, virology, neuroscience, cardiology, geriatrics, as well as gastrointestinal and liver diseases.
Icahn Mount Sinai offers highly competitive MD, PhD, and Master’s degree programs, with current enrollment of approximately 1,300 students. It has the largest graduate medical education program in the country, with more than 2,000 clinical residents and fellows training throughout the Health System. In addition, more than 550 postdoctoral research fellows are in training within the Health System.
A culture of innovation and discovery permeates every Icahn Mount Sinai program. Mount Sinai’s technology transfer office, one of the largest in the country, partners with faculty and trainees to pursue optimal commercialization of intellectual property to ensure that Mount Sinai discoveries and innovations translate into healthcare products and services that benefit the public.
Icahn Mount Sinai’s commitment to breakthrough science and clinical care is enhanced by academic affiliations that supplement and complement the School’s programs.
Through the Mount Sinai Innovation Partners (MSIP), the Health System facilitates the real-world application and commercialization of medical breakthroughs made at Mount Sinai. Additionally, MSIP develops research partnerships with industry leaders such as Merck & Co., AstraZeneca, Novo Nordisk, and others.
The Icahn School of Medicine at Mount Sinai is located in New York City on the border between the Upper East Side and East Harlem, and classroom teaching takes place on a campus facing Central Park. Icahn Mount Sinai’s location offers many opportunities to interact with and care for diverse communities. Learning extends well beyond the borders of our physical campus, to the eight hospitals of the Mount Sinai Health System, our academic affiliates, and globally.
-------------------------------------------------------
* Mount Sinai Health System member hospitals: The Mount Sinai Hospital; Mount Sinai Beth Israel; Mount Sinai Brooklyn; Mount Sinai Morningside; Mount Sinai Queens; Mount Sinai South Nassau; Mount Sinai West; and New York Eye and Ear Infirmary of Mount Sinai.
END