(Press-News.org) Generative Artificial Intelligence (Gen AI) can rapidly and accurately screen patients for clinical trial eligibility, according to a new study from Mass General Brigham researchers. Such technology could make it faster and cheaper to evaluate new treatments and, ultimately, help bring successful ones to patients.
Investigators assessed the accuracy and cost of a Gen AI process they named RAG-Enabled Clinical Trial Infrastructure for Inclusion Exclusion Review (RECTIFIER), that identifies patients who meet criteria for enrollment in a heart failure trial based on their medical records. For criteria that require reviewing patient notes, they found that RECTIFIER screened patients more accurately than disease-trained research coordinators who typically conduct screening — and for a fraction of current costs. The results of the study were published in NEJM AI.
“We saw that large language models hold the potential to fundamentally improve clinical trial screening,” said co-senior author Samuel (Sandy) Aronson, ALM, MA, executive director of IT and AI Solutions for Mass General Brigham Personalized Medicine and senior director of IT and AI Solutions for the Accelerator for Clinical Transformation. “Now the difficult work begins to determine how to integrate this capability into real world trial workflows in a manner that simultaneously delivers improved effectiveness, safety, and equity.”
Clinical trials enroll people that meet specific criteria, such as age, diagnoses, key health indicators and current or past medications. These criteria help researchers ensure they enroll participants who are representative of those expected to benefit from the treatment. Enrollment criteria also help researchers avoid including patients who have unrelated health problems or are taking medications that could interfere with the results.
Co-lead author Ozan Unlu, MD, a fellow in Clinical Informatics at Mass General Brigham and a fellow in Cardiovascular Medicine at Brigham and Women's Hospital, said, “Screening of participants is one of the most time-consuming, labor-intensive, and error-prone tasks in a clinical trial.”
The research team, part of the Mass General Brigham Accelerator for Clinical Transformation, tested the ability of the AI process to identify patients eligible for The Co-Operative Program for Implementation of Optimal Therapy in Heart Failure (COPILOT-HF) trial, which recruits patients with symptomatic heart failure and identifies potential participants based on electronic health record (EHR) data. The researchers designed 13 prompts to assess clinical trial eligibility. They tested and tweaked those prompts using medical charts of a small group of patients, before applying them to a dataset of 1,894 patients with an average of 120 notes per patient. They then compared the screening performance of this process to those of study staff.
The AI process was 97.9% to 100% accurate, based on alignment with an expert clinician’s “gold standard” assessment of whether the patients met trial criteria. In comparison, study staff assessing the same medical records were slightly less precise than AI, with accuracy rates between 91.7% and 100%.
The researchers estimated the AI model costs about $0.11 to screen each patient. The authors explain that this is orders of magnitude less expensive compared to traditional screening methods.
Co-senior author Alexander Blood, MD, a cardiologist at Brigham and Women’s Hospital and associate director of the Accelerator for Clinical Transformation, noted that using AI in clinical trials could speed up the time it takes to determine whether a therapy is effective. “If we can accelerate the clinical trial process, and make trials cheaper and more equitable without sacrificing safety, we can get drugs to patients faster and ensure they are helping a broad population,” Blood said.
The researchers noted that AI can have risks that should be monitored when it is integrated to routine workflows. It could introduce bias and miss nuances in medical notes. Plus, a change in how data is captured in a health system could significantly impact the performance of AI.
For these reasons, any study using AI to screen patients needs to have checks in place, the authors concluded. Most trials have a clinician who doublechecks the participants whom the study staff deem eligible for a trial, and the researchers recommended that this final check continue with AI screening.
“Our goal is to prove this works in other disease areas and use cases while we expand beyond the walls of Mass General Brigham,” added Blood.
Authorship: In addition to Unlu, Blood and Aronson, Mass General Brigham authors include co-first author Jiyeon Shin (BWH, MGB), Charlotte Mailly (BWH, MGB), Michael Oates (BWH, MGB), Michaela Tucci (BWH), Matthew Varugheese (BWH), Kavishwar Wagholikar (BWH), Fei Wang (BWH, MGB), and Benjamin Scirica (BWH).
Disclosures: Microsoft provided complimentary access to Azure OpenAI GPT-4V. Microsoft had no access to the data used and had no involvement in the analysis, interpretation of data, or writing of the study.
Paper cited: Unlu, O. et al. “Retrieval Augmented Generation–Enabled GPT-4 for Clinical Trial Screening” NEJM AI. DOI: 10.1056/AIoa2400181
###
About Mass General Brigham
Mass General Brigham is an integrated academic health care system, uniting great minds to solve the hardest problems in medicine for our communities and the world. Mass General Brigham connects a full continuum of care across a system of academic medical centers, community and specialty hospitals, a health insurance plan, physician networks, community health centers, home care, and long-term care services. Mass General Brigham is a nonprofit organization committed to patient care, research, teaching, and service to the community. In addition, Mass General Brigham is one of the nation’s leading biomedical research organizations with several Harvard Medical School teaching hospitals. For more information, please visit massgeneralbrigham.org.
END
The first generation of lithium-ion batteries for electric vehicles has been a remarkable success story. Yet, the question arises: What changes to battery materials will spur further advances to extend driving range and lower costs?
A better positive electrode, or cathode, for lithium-ion batteries has been the focus of intense past research. The cathode is one of the main components in batteries. Several candidates for cathode materials offer the prospect of batteries with much higher energy storage, leading to longer driving range. However, the capacity, or amount of current flowing out within a given time, tends to decline rapidly with charge-discharge cycling for reasons ...
PHILADELPHIA (June 17, 2024) - In a recently published opinion piece in BMJ Open, “Rhetoric of Research: A Call for Renaming the Clinical Research Partnership,” authors from Penn Nursing and Georgetown University School of Nursing, present a compelling argument for rethinking the language used to describe participants in clinical research. The opinion calls for a shift from the traditional term “patient participant” to “participant partner,” emphasizing the crucial role of participants in ...
SAN ANTONIO — June 17, 2024 —Southwest Research Institute (SwRI) today celebrated the groundbreaking of the Center for Accelerating Materials and Processes (CAMP), a new facility that will support research and development for tomorrow’s high-speed aerospace engines.
“This project will help ensure the U.S. is a leader in high-speed propulsion research and development,” said Dr. Barron Bichon, director of SwRI’s Materials Engineering Department. “SwRI is committed to advancing this vital technology on behalf of Texas and the nation.”
Market forces including growth in global defense, air travel, ...
Since the very first instant after the Big Bang the Universe has been expanding. This means that the early Universe was considerably smaller and early-formed galaxies were more likely to interact and merge. Galaxy mergers fuel the formation of quasars — extremely luminous galactic cores where gas and dust falling into a central supermassive black hole emit enormous amounts of light. So when looking back at the early Universe astronomers would expect to find numerous pairs of quasars in close proximity to each other as their host galaxies undergo mergers. However, they have been surprised ...
Roughly 50 families scattered across the world share ultra-rare variants in a particular gene. Silent for years, the inherited mutations make themselves known when patients reach the fourth decade of life. Changes in vision start a cascade of symptoms. Five to 20 years later, the illness is fatal.
Researchers at Washington University School of Medicine in St. Louis have dedicated many years to understanding the rare condition known as retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations, or RVCL-S, with the aim of developing a treatment ...
A new smartphone face-screening tool could help paramedics to identify stroke in seconds – much sooner and more accurately than is possible with current technologies.
Strokes, which affect millions of people globally, occur when the blood supply to part of the brain is interrupted or reduced, which prevent brain tissue from getting oxygen and nutrients. A few minutes of delay can result in permanent damage to the brain cells.
A team of biomedical engineers at RMIT University developed the AI capabilities behind the software technology and has published their results ...
WASHINGTON, June 17, 2024 — L-DOPA is the best drug we have for Parkinson’s disease, but its molecular mirror image, D-DOPA, causes dangerous side effects. Making L-DOPA without also making D-DOPA is surprisingly hard and requires a specific kind of molecule to pull off. But that specific molecule must be made from a different and equally specific molecule. In this video, our host, George Zaidan, explains how one of the winners of the 2001 Nobel Prize in Chemistry pulled it off, and why "chiral synthesis," as it's called, is really just turtles all the way down. https://youtu.be/_cb09XB07LQ?si=BuMEI5fOuHmuQlkZ
Reactions is a video series ...
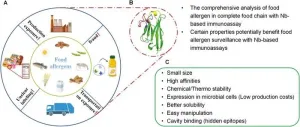
Recent advancements show nanobodies from camelid antibodies excel in food allergen detection with superior stability, specificity, and cost-effectiveness. This innovative approach aims to improve accuracy and efficiency, crucial for preventing severe allergic reactions. The study highlights nanobodies' potential in reliable immunoassays, addressing rising food allergies and enhancing safety measures.
Food allergies pose significant health risks, affecting millions worldwide, with the prevalence rising over the past decades. Traditional detection methods, such as monoclonal and polyclonal antibodies, are often costly, labor-intensive, and prone to cross-reactions. The need for ...
Researchers from the Substance Abuse and Mental Health Services Administration (SAMHSA), the National Institutes of Health’s (NIH) National Institute on Drug Abuse (NIDA), Centers for Medicare & Medicaid Services (CMS), and the Centers for Disease Control and Prevention (CDC) found that among a cohort of 137,000 Medicare beneficiaries who experienced a nonfatal overdose in 2020, almost 24,000 (17.4%) experienced a subsequent nonfatal overdose, and about 1,300 (1%) died from overdose in the following year. Results were published today in JAMA Internal Medicine, identifying both effective interventions and significant gaps in care.
“People who have experienced ...
KEY TAKEAWAYS
Mass General Brigham researchers analyzed 4,400 cognitively unimpaired adults with amyloid imaging, finding increased amyloid in those who reported that their mothers had symptoms of Alzheimer’s disease (AD).
Increased amyloid, a biomarker of AD, was also found in those with a history of the disease on both sides of their family and in those whose fathers had an early onset of symptoms.
The study suggests that a person’s maternal versus paternal family history could ...