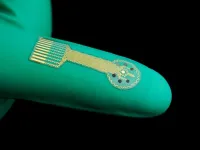
Chronic wounds, which include diabetic ulcers, surgical wounds, pressure injuries, and other problems, are deadlier than many people realize. Patients with chronic wounds have a five-year survival rate around 70%, worse than that of breast cancer, prostate cancer and other serious diseases. Treating wounds is also expensive, costing an estimated $28 billion each year in the U.S. alone.
A team of researchers from the Keck School of Medicine of USC and the California Institute of Technology (Caltech) is developing a series of cutting-edge technologies to revolutionize wound care, including smart bandages that would automatically sense and respond to changing conditions inside a wound. These high-tech dressings would provide continuous data on healing and potential complications, including infections or abnormal inflammation, and could deliver medications or other treatments in real time.
Supported in part by the National Institutes of Health, the USC-Caltech team has developed and tested a smart bandage in animal models in a proof-of-concept study. They have now published a review of that research, as well as other cutting-edge wound monitoring and treatment work being done around the world, in the journal Nature Materials. The review also assesses challenges and next steps for delivering these technologies to patients, including the outlook for regulatory approval and commercialization.
“We’re creating a new kind of ‘cyber skin’ that can help these wounds heal, while measuring and managing them along the way,” said co-senior author David G. Armstrong, PhD, DPM, a professor of surgery and neurological surgery at the Keck School of Medicine and co-director of the Southwestern Academic Limb Salvage Alliance (SALSA). “This paper combines these recent insights to chart a way forward in the wound healing space, so that we can move quickly to help our patients recover.”
To refine smart bandage technology, Armstrong and his team have leveraged new breakthroughs from the fields of materials science, nanotechnology, digital health and more. Other recent changes, including more funding for wound healing research and an improved path toward regulatory approval, have also paved the way for progress.
“We have been developing next-generation smart bandages that can wirelessly monitor crucial metabolic and inflammatory biomarkers in wound fluids,” said Wei Gao, PhD, an assistant professor of medical engineering at Caltech and co-senior author of the paper. “Going forward, these interdisciplinary collaborations among scientists, engineers and clinical experts—with patients at the center—will play a crucial role in better wound care outcomes.”
Next-generation smart dressings
While acute wounds progress through a typical process of injury, inflammation and healing, chronic wounds are more complex and less predictable. They carry a higher risk of infection, can take longer to heal, and may lead to amputation or life-threatening complications, such as sepsis.
One potential remedy is new smart bandage technology that can aid—and even participate in—the healing process. Instead of applying a passive dressing to a patient’s wound, clinicians could soon use wireless technology that detects inflammation, infections or problems with blood flow, then alerts patients and health care providers via Bluetooth while administering real-time treatment. Armstrong and his team have tested the new technology in animal models, with promising results.
“This closed-loop system can identify a problem, diagnose it automatically, and deliver a solution, all with patient and clinician oversight,” he said.
Smart bandages are built using a variety of cutting-edge materials, including bioelectronic materials, which can help with healing by delivering electrical stimulation to tissues and cells. Many incorporate advanced hydrogels, which are soft, flexible, and capable of storing and releasing drugs in response to pH, temperature or other environmental factors.
Next-generation dressings also contain various types of sensors that can detect changes in the microenvironment of a wound. Electrochemical sensors can measure the presence of proteins, antibodies, nutrients and electrolytes, while optical sensors can monitor temperature, pH and oxygen levels. Imaging sensors, including photography, ultrasound and fluorescence imaging, can detect bacterial infections and measure the depth and volume of a wound to track healing progress.
In their review of new technologies, the researchers point to several hurdles that require attention before smart bandages can enter standard medical practice. For one, many medical systems use outdated approaches to wound care, such as visually assessing and classifying wounds without standardized criteria (which can result in imprecise or unreliable evaluations). Integrating smart bandages, therefore, would require a significant overhaul of the field’s current standards.
“While the idea of a dressing that would help us how we need when we need it makes sense to us, it also has to make sense to our colleagues at the FDA,” Armstrong said.
Obtaining regulatory approval for smart bandages from the U.S. Food and Drug Administration (FDA) is also complex. The FDA offers flexibility for approval of wound care products that combine multiple therapies (for example, delivering both antibiotics and electrical stimulation). But obtaining special approval requires researchers to collect a large quantity of preclinical and clinical data. This is the ongoing goal of the USC-Caltech research team.
Measuring and managing wounds
Once data is collected by a smart bandage, it can be processed and analyzed with machine learning tools that allow for fast and efficient monitoring and care, either in the doctor’s office or from a distance.
Armstrong likens this new approach to detecting high cholesterol in the early stages of heart disease, enabling treatment with a statin.
“What’s amazing is that in wound healing, we haven’t been using those interim measures. All we’ve done is the equivalent of measuring someone in the middle of a heart attack,” he said. “Developing these interim companion diagnostics is critical.”
More responsive wound care can not only save lives, it can also improve the quality of life for many patients. About half of people with chronic wounds meet the diagnostic criteria for clinical depression and many face serious challenges with mobility, pain and wound care on a daily basis.
“We want to maximize ulcer-free, hospital-free and activity-rich days for our patients,” Armstrong said.
Next, he and his team are studying a new approach to wound care that uses ultrasound technology to guide the delivery of gene therapy treatment. The goal is to stimulate blood vessel growth in calf muscles, which could help reduce the risk for amputation in patients with leg ulcers.
About this research
In addition to Armstrong and Gao, the study’s other authors are Chia-Ding Shih from the Department of Surgery, Keck School of Medicine of USC; Canran Wang and Ehsan Shirzaei Sani from the Andrew and Peggy Cherng Department of Medical Engineering, California Institute of Technology; Chwee Teck Lim from the National University of Singapore; and Joseph Wang from the Department of Nanoengineering, University of California San Diego.
This work was supported by the National Science Foundation [2145802]; the National Institutes of Health [R01HL155815, R21DK13266]; the Army Research Office [W911NF-23-1-0041]; the American Cancer Society [RSG-21-181-01-CTPS]; the Office of Naval Research [N00014-21-1-2483, N00014-21-1-2845]; and Heritage Medical Research Institute.
END