This work, led by Andrew F. Stewart, MD, Irene and Dr. Arthur M. Fishberg Professor of Medicine and Director of the Mount Sinai Diabetes, Obesity and Metabolism Institute, began at the Icahn School of Medicine at Mount Sinai in 2015. The studies were a team effort. Adolfo Garcia-Ocaña, PhD, formerly a professor at Mount Sinai and who is now at City of Hope, a leading research center for diabetes and one of the largest cancer research and treatment organizations in the United States, and is the Ruth B. and Robert K. Lanman Chair in Gene Regulation and Drug Discovery Research and chair of the Department of Molecular & Cellular Endocrinology, and his research team designed the studies and performed the novel, extensive and detailed animal transplant and drug treatment models using beta cells from donors. Final studies took place at City of Hope in 2023.
For the study, the natural product harmine, which is found in some plants, was combined with a widely used class of type 2 diabetes therapy called GLP1 receptor agonists. Researchers transplanted a small number of human beta cells into mice that had no immune system and that also served as a standard model of type 1 and type 2 diabetes; these mice were treated with the combination therapy and their diabetes was rapidly reversed. Strikingly, human beta cell numbers increased by 700 percent over three months with this drug combination.
“This is the first time scientists have developed a drug treatment that is proven to increase adult human beta cell numbers in vivo. This research brings hope for the use of future regenerative therapies to potentially treat the hundreds of millions of people with diabetes,” said Dr. Garcia-Ocaña, the paper’s corresponding author.
“It has been remarkable to watch this story unfold over the past 15 years,” said Dr. Stewart, who, along with Peng Wang, PhD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at Icahn Mount Sinai, conceived of and performed the initial high-throughput drug screen that led to the discovery of harmine described in Nature Medicine in 2015. “The steady progression from the most basic human beta cell biology, through robotic drug screening and now moving to human studies, illustrates the essential role for physician-scientists in academia and pharma.”
Growing New Beta Cells
More than 10 percent of the world’s adult population has diabetes, a disease defined by high blood sugar levels. In both type 1 and type 2 diabetes, a reduction in both the quantity and quality of insulin-producing beta cells causes high blood sugar. Unfortunately, none of the many commonly used diabetes therapies are able to increase human beta cell numbers, and therefore cannot completely reverse diabetes.
Fortunately, most people with diabetes have some residual beta cells, which is what inspired the research team to search for ways to restore their numbers. The team had previously shown that several different inhibitors of an enzyme in beta cells called DYRK1A can induce the proliferation of adult human beta cells in a tissue culture dish for a few days. But prior to this study, no one had shown the ability to expand human beta cells numbers in vivo in human islet grafts used in an animal model over many months.
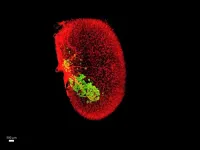
To accurately measure the mass of human beta cells in the islet grafts, the team turned to Sarah A. Stanley, MBBCh, PhD, Associate Professor of Medicine (Endocrinology, Diabetes and Bone Disease), and Neuroscience, at Icahn Mount Sinai. Using an advanced laser microscopy tool called iDISCO+ that effectively makes biological tissue transparent, Dr. Stanley saw that beta cell mass was dramatically increased through mechanisms that included enhanced proliferation, function, and survival of the human beta cells. The technology allowed for accurate and rigorous quantitative assessment of engrafted human beta cells for the first time.
Translating Results to the Clinic
The Mount Sinai team recently completed a phase 1 clinical trial of harmine in healthy volunteers to test its safety and tolerability. At the same time, Robert J. DeVita, PhD, Professor of Pharmacological Sciences and Director of the Marie-Josée and Henry R. Kravis Drug Discovery Institute at Mount Sinai, has developed next-generation DYRK1A inhibitors. Mount Sinai is conducting studies to test these in humans for potential toxicity risks and estimate dosing for clinical trials, and is planning to initiate first-in-human trials with independent research teams next year. Mount Sinai owns an extensive patent portfolio covering these technologies.
Researchers also want to address the fact that in patients with type 1 diabetes, the immune system will continue to kill new beta cells. At City of Hope, Dr. Garcia-Ocaña and colleague Alberto Pugliese, MD, Samuel Rahbar Chair in Diabetes & Drug Discovery, chair of the Department of Diabetes Immunology, and director of The Wanek Family Project for Type 1 Diabetes within the Arthur Riggs Diabetes & Metabolism Research Institute, plan to test inducers of beta cell regeneration together with immunomodulators that regulate the immune system. Their goal is for the combination to allow new beta cells to thrive and improve insulin levels.
“Our studies pave the way for moving DYRK1A inhibitors into human clinical trials and it's very exciting to be close to seeing this novel treatment used in patients,” Dr. Garcia-Ocaña said. “There is nothing like this available to patients right now.”
The work outlined in the Science Translational Medicine paper was funded by grants from the National Institutes of Health (NIH), the National Institute of Diabetes Digestive and Kidney Disease, and BreakthroughT1D (formerly JDRF); as well as from philanthropic donations to Mount Sinai, support from The Wanek Family Project for Type 1 Diabetes at City of Hope, and additional generous philanthropic gifts.
Other critical members of the team include Mount Sinai’s Carolina Rosselot, PhD; Yansui Li, PhD; and Alexandra Alvarsson, PhD. Additional City of Hope authors on the paper are Geming Lu, MD, assistant research professor, and Randy Kang, BS, senior research associate, who are both members of Dr. Garcia-Ocaña’s lab.
Drs. Stewart and DeVita are named co-inventors on patent applications for DYRK1A inhibitors, such as harmine, for the treatment of diabetes. These patent applications are filed through the Icahn School of Medicine at Mount Sinai and are currently unlicensed.
# # #
About City of Hope
City of Hope's mission is to make hope a reality for all touched by cancer and diabetes. Founded in 1913, City of Hope has grown into one of the largest cancer research and treatment organizations in the U.S. and one of the leading research centers for diabetes and other life-threatening illnesses. City of Hope research has been the basis for numerous breakthrough cancer medicines, as well as human synthetic insulin and monoclonal antibodies. With an independent, National Cancer Institute-designated comprehensive cancer center at its core, City of Hope brings a uniquely integrated model to patients spanning cancer care, research and development, academics and training, and innovation initiatives. City of Hope’s growing national system includes its Los Angeles campus, a network of clinical care locations across Southern California, a new cancer center in Orange County, California, and cancer treatment centers and outpatient facilities in the Atlanta, Chicago and Phoenix areas. City of Hope’s affiliated group of organizations includes Translational Genomics Research Institute and AccessHopeTM. For more information about City of Hope, follow us on Facebook, X, YouTube, Instagram and LinkedIn.
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, with 48,000 employees working across eight hospitals, more than 400 outpatient practices, more than 600 research and clinical labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time—discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 9,000 primary and specialty care physicians and 11 free-standing joint-venture centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida. Hospitals within the System are consistently ranked by Newsweek’s® “The World’s Best Smart Hospitals, Best in State Hospitals, World Best Hospitals and Best Specialty Hospitals” and by U.S. News & World Report's® “Best Hospitals” and “Best Children’s Hospitals.” The Mount Sinai Hospital is on the U.S. News & World Report® “Best Hospitals” Honor Roll for 2023-2024.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Twitter and YouTube.
END