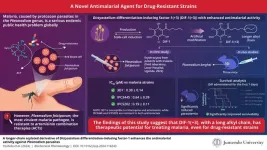
Malaria remains a serious health issue globally, especially in Africa. The disease is caused by protozoan parasites in the Plasmodium genus. In 2021, there were 247 million cases of malaria and 619,000 deaths reported worldwide. At present, the first line of treatment against malaria is artemisinin combination therapy (ACT) and the administration of artemisinin derivatives in combination with other drugs. After the introduction of ACTs in Africa, which accounts for more than 90% of the world’s malaria cases, the number of deaths due to malaria greatly declined in the mid-2000s. However, Plasmodium falciparum, the most virulent malaria parasite, is resistant to ACT and has been spreading across Asia and some African countries. Therefore, new antimalarial agents that are effective against drug-resistant parasites are urgently needed.
Fortunately, a team of researchers led by Professor Toshihiro Mita from the Department of Tropical Medicine and Parasitology, Faculty of Medicine at Juntendo University, Japan, and Dr. Yuzuru Kubohara from the Laboratory of Health and Life Science, Graduate School of Health and Sports Science at Juntendo University has developed a promising new antimalarial agent, DIF-1(+3), which is a derivative of Dictyostelium differentiation-inducing factor-1 (DIF-1). Prof. Mita explains, “Dictyostelium discoideum, a cellular slime mold, has been utilized as a model organism for decades. Recently, it has gained attention as a potential resource for drug discovery. In 2021, we discovered that Dictyostelium DIF-1 and its derivatives exhibited antimalarial properties. Now, we have successfully synthesized a more potent derivative, DIF-1(+3).”
The team also included Assistant Professor Naoko Yoshida, also from Juntendo University, and Professor Haruhisa Kikuchi from Keio University, Japan. Their study was made available online on April 30, 2024, and published in Volume 225 of the journal Biochemical Pharmacology in July 2024.
The researchers synthesized DIF-1(+3) and compared its antimalarial activity to other DIF derivatives through in vitro and in vivo tests. In the in vitro tests, DIF-1(+3) was tested against three laboratory clones of Plasmodium falciparum, two of which were resistant to chloroquine and artemisinin, and 13 natural isolates of the same, obtained from patients with malaria in 2022 at the field laboratory of Lacor Hospital in Uganda. In the in vivo tests, the efficacy of DIF-1(+3) was tested in mice infected with Plasmodium berghei, a malaria parasite that affects rodents.
The experiments revealed that DIF-1(+3) had significantly stronger growth inhibitory effects against the three laboratory clones, compared to DIF-1(+2), which was the most active derivative found up until this study. Similar results were observed for the natural Ugandan isolates. In the in vivo study, DIF-1(+3) almost completely suppressed parasite growth during the treatment period and significantly increased the survival time of the infected mice compared to those treated with DIF-1(+2). The researchers attributed this enhanced antimalarial activity to the longer alkyl chain in DIF-1(+3) compared to other DIF derivatives.
Highlighting the importance of their study, Dr. Kubohara says, “Our latest compound proved to be as effective against drug-resistant malaria as it was against susceptible strains. This suggests that antimalarial drugs based on DIF compounds may offer more treatment options in endemic areas where artemisinin-resistant malaria is spreading.” The high efficacy of DIF-1(+3) against artemisinin and other existing antimalarial agents suggests that it may have a different mechanism of action, requiring further research.
Looking ahead, Prof. Mita concludes, “Further research may identify the target molecule for antimalarial action, which could lead to new drug discovery research with a high impact on related fields. By using this new compound as a seed to advance drug discovery, we could create a tool that can help eliminate malaria. WHO and other global health organizations promoting malaria control will also benefit from this research”
Reference
Authors
Naoko Yoshidaa, Haruhisa Kikuchib, Makoto Hiraia, Betty Balikagalaa, Denis A. Anywarc, Hikari Takad, Naoko Kagad, Yoshiki Miurad, Naoyuki Fukudaa, Emmanuel I. Odongo-Aginyac, Yuzuru Kuboharae,*, and Toshihiro Mitaa,*
Title of original paper
A longer-chain acylated derivative of Dictyostelium differentiation-inducing factor-1 enhances the antimalarial activity against Plasmodium parasites
Journal
Biochemical Pharmacology
DOI
10.1016/j.bcp.2024.116243
Affiliations
a Department of Tropical Medicine and Parasitology, Faculty of Medicine, Juntendo University, Japan
b Division of Natural Medicines, Faculty of Pharmacy, Keio University, Japan
c Faculty of Medicine, Gulu University, Uganda
d Laboratory of Proteomics and Biomolecular Science, Biomedical Research Core Facilities, Faculty of Medicine, Juntendo University, Japan
e Laboratory of Health and Life Science, Graduate School of Health and Sports Science, Juntendo University, Japan
About Professor Toshihiro Mita
Dr. Mita is currently a Professor and the Chairman of the Department of Molecular and Cellular Parasitology, at Juntendo University School of Medicine. He graduated from Nagasaki University, Faculty of Medicine in 1990 and started his professional career as gastroenterologist. During his research career, he has worked in the field of evolution and the genetics of drug resistance of malaria parasites. Dr. Mita first discovered and reported the phenomenon of recovery of chloroquine sensitivity seven years after the discontinuation of its usage for malaria treatment in Malawi in 2003. He holds a medical doctorate and two specializations (Fellow of the Japanese Society of Internal Medicine and the Japanese Society of Digestive Endoscopy). In 2016, he was appointed as the tentative member of the WHO Evidence Review Group meeting on the emergence and spread of drug-resistant malaria. He has over 70 publications with over 1500 citations. His research interests include tropical medicine, malaria, drug resistance, genetics, molecular epidemiology.
END