(Press-News.org) The results of the PrEPVacc HIV vaccine trial conducted in Eastern and Southern Africa, which ran between 2020 and 2024, show conclusively that neither of the two experimental vaccine regimens tested reduced HIV infections among the study population.
Vaccinations in the PrEPVacc trial were stopped in November 2023 (and publicly announced in December 2023) when it became clear to independent experts monitoring the study data that there was little or no chance of the vaccines demonstrating efficacy in preventing HIV acquisition.
The PrEPVacc vaccine trial results, announced today at AIDS 2024 in Munich, Germany, report more infections in the two vaccine arms than in the placebo arms, but the researchers say they cannot draw a definitive conclusion about what this means because the statistical ‘confidence intervals’ for the comparison are so wide, indicating a high degree of uncertainty.
The researchers also highlight that the rate of HIV infection observed in the placebo group was unusually low and does not appear to be explained by a difference in the use of condoms or pre-exposure prophylaxis (PrEP).
PrEPVacc is discussing with other groups worldwide further immunological analyses that could help to explain the differences in HIV infection rates between the vaccine and placebo groups.
Across South Africa, Tanzania and Uganda, the countries where PrEPVacc has conducted its trial, UNAIDS estimates that in 2022, a total of 10.7 million people were living with HIV and 244,000 adults and children were newly diagnosed with HIV. At the time its participants exited from the study, PrEPVacc was the only ongoing HIV vaccine efficacy trial in the world, and there are currently no other HIV efficacy trials underway or in the pipeline. PrEPVacc is the first HIV vaccine efficacy trial to be conducted in East African countries.
The PrEPVacc study, led by African researchers with support from European colleagues, is three trials in one. In its phase IIb exploratory efficacy trial, it tested two different vaccine regimens to see if either could prevent HIV infection in populations who may be vulnerable to acquiring HIV. During the period that participants received the first three vaccinations, a new form of oral pre-exposure prophylaxis (PrEP) was also tested against the existing standard for PrEP to see whether it was as effective at preventing HIV infections. PrEPVacc’s oral PrEP results are separate from the vaccine results and will be announced later in 2024.
Study approach in brief
Across four sites in Masaka, Uganda; Mbeya and Dar es Salaam, Tanzania; and Durban, South Africa, PrEPVacc enrolled 1,512 healthy adults aged 18-40 years who reported behaviours that made them more vulnerable to acquiring HIV. The Masaka and Durban sites enrolled men and women, and the Mbeya and Dar es Salaam sites enrolled only women. Across all sites, 13% of participants were men, and 87% were women.
PrEPVacc tested two different combinations of HIV vaccines and compared each to a placebo (saline water) in a 1:1:1 randomisation. One regimen combined a DNA vaccine (DNA-HIV-PT123) with a protein vaccine (AIDSVAX B/E), and the other combined the same DNA vaccine, a modified non dividing virus vector (MVA-CMDR) and a protein-based vaccine (CN54gp140). The schedule had four vaccine injection visits, three over approximately six months and a fourth a year after enrolment.
All participants were offered and counselled on the benefits of oral PrEP throughout the trial. From enrolment to two weeks after their third vaccine visit PrEP was offered as one of two study drugs, and after that, it was locally sourced. In addition, they received tailored HIV risk counselling as well as information on how best to incorporate and adhere to PrEP in combination prevention against HIV.
PrEPVacc’s Investigators successfully completed the trial, meeting the ethical requirements, implementing good community engagement and participatory practices (GPP) and completing multiple studies as part of its integrated social science programme.
Investigators encountered challenges in successfully recruiting and enrolling in clinical trials during the COVID-19 pandemic and in dealing with the supply of the MVA vaccine. This was mitigated when PrEPVacc changed the randomisation of enrolled participants from 1:1:1 DNA/AIDSVAX B/E, DNA/MVA-CMDR/CN54gp140 and placebo arms, to 1:1 between DNA/AIDSVAX B/E and placebo arms, and discontinued enrolment to the MVA-CMDR/CN54gp140-combination vaccine arm in all sites by June 2022.
PrEPVacc’s primary vaccine efficacy analysis was designed to give the vaccines the best chance of showing effectiveness by only considering those participants who received three or more injections from the combination or placebo group that they were randomised to, which is when all vaccinated participants were expected to achieve peak immune responses.
Small numbers of infections in the vaccine trials
In the primary vaccine analysis, of those who received at least three injections of the DNA/AIDSVAX B/E combination, 11 out of 532 participants acquired HIV, an incidence rate of 1.73 infections per 100 person years (95% confidence interval of 0.96 to 3.12) compared to 3 out of 523 participants who received the placebo, an incidence rate of 0.48 infections per 100 person years (95% confidence interval of 0.15 to 1.48). The confidence interval for the adjusted hazard ratio is 1.03 to 13.20, and the p-value for this difference is 0.045.
In the primary vaccine analysis, of those who received the MVA-CMDR/CN54gp140 combination, 9 out of 244 participants acquired HIV, an incidence rate of 2.38 infections per 100 person years (95% confidence interval of 1.24 to 4.57) compared to 2 out of 251 participants who received the placebo, an incidence rate of 0.51 infections per 100 person years (95% confidence interval of 0.13 to 2.02). This result's adjusted hazard ratio confidence interval is 0.98 to 21.12, and the p-value for this difference is 0.052.
These results mean that neither vaccine combination offered any protective effect, and the researchers say that the confidence intervals around the hazard ratios are so wide they cannot draw a definitive conclusion about what the higher number of infections in the vaccine arms means.
In advance of public release, PrEPVacc has begun sharing these results with the participants and communities who have been partners in the trial and will now inform individual participants which vaccine or placebo group they were in.
No definitive conclusion from finding more infections in the vaccine arm
From PrEPVacc’s Registration Cohort observational study between 2018 and 2023, an HIV incidence rate of 2.9 infections per 100 person years (95% confidence intervals of 2.4 to 3.5) was observed at the trial study sites. PrEPVacc investigators would have expected to see a similar HIV incidence in the vaccine trial placebo group.
The observed HIV incidence rates in the matched placebo groups were 0.48 and 0.51 per 100 person years, much lower than expected by the investigators and with wide confidence intervals due to the low number of infections. These much lower than expected incidence rates in the placebo arms add to the uncertainty.
For comparison, the recent announcement from the PURPOSE 1 study of injectable and oral forms of PrEP, which took place at three sites in Uganda and 25 in South Africa, reported 16 infections among 1,068 women in its Truvada group (an incidence of 1.69 per 100 person-years) and 39 infections among 2,136 women in its Descovy group (an incidence of 2.02 per 100 person-years). The reported incidences in PURPOSE 1 are much closer to what was expected in PrEPVacc’s placebo arm.
While the investigators regard it as good news that HIV incidence fell in the study populations during the trial – continuing a trend seen in the observational study – it means that uncertainty about the PrEPVacc vaccine result is high.
The investigators cannot immediately explain the relatively low incidence of HIV in the placebo arm. They have ruled out statistical errors and differences in self-reported PrEP adherence use of condoms, and risk behaviours between the groups.
Safety of vaccines demonstrated in earlier trials
The vaccine candidates tested in PrEPVacc have been evaluated in various combinations in multiple phase I/II clinical trials in the US, Europe and Africa. They have demonstrated their safety and ability to induce immune responses. It is not scientifically possible to get HIV infections from the vaccines that were used in this study.
PrEPVacc’s Trial Safety Group reviews the safety information of participants twice a month and considers any incidents or side effects that may arise while participants are on the trial. The Trial Safety Group has had no concerns about the side effects of the vaccines tested in PrEPVacc.
PrEPVacc participants to be supported with ongoing testing and counselling
During the trial, counselling, promotion and provision of PrEP were emphasised at every visit to all participants, with active follow-up regarding use. All participants have now completed study visits and have received counselling on other methods of HIV prevention known to be effective.
Follow-up HIV testing is already available through the centres, and PrEPVacc will continue to counsel and promote PrEP and facilitate referral for care in case of infections. Referral to care has been facilitated for those who have acquired HIV.
At the study's exit, about a quarter of participants allocated to an active vaccine regimen displayed evidence of Vaccine-Induced Sero-Positivity (VISP). VISP occurs when the body has produced antibodies after receiving an HIV vaccine, and subsequent HIV test results can be positive even if an individual is not infected with HIV.
PrEPVacc has always intended to offer a service to participants with VISP to ensure they receive an accurate diagnosis, and PrEPVacc will follow all participants, with the aim of conducting HIV testing twice yearly for at least two years to monitor the trend in infections, and support access to care and PrEP.
Reactions from trial leaders, community member and independent expert
PrEPVacc’s Trial Director, Dr Eugene Ruzagira, based at the MRC/UVRI & LSHTM Uganda Research Unit in Uganda, who presented the results to the AIDS 2024 conference today, said:
“Throughout PrEPVacc, we have put our participants and their communities foremost and ensured their safety. We stopped the vaccine trials in November 2023 as soon as we had evidence that the vaccines could not be shown to be effective. We will continue to support our participants with counselling, testing and access to available prevention and care options.
“Our participants’ dedication to this study has been exemplary. They and their communities should be very proud of their efforts and their important contributions to the global effort to prevent HIV.
“The positive news for our communities is that repeated risk reduction counselling and use of proven HIV prevention tools helps people navigate their risks better. The number of new infections has fallen throughout the six years we have been monitoring the HIV incidence in each study community.”
“We can be proud of the efforts we have made to grow capacity through PrEPVacc that have enabled our participation in the BRILLIANT consortium. This consortium builds upon PrEPVacc’s legacy of African leadership in HIV prevention research.”
“We look forward to the findings from PrEPVacc’s integrated social science research and the results of the PrEP study in PrEPVacc to come later this year.”
Professor Sheena McCormack, PrEPVacc Project Lead based at the Medical Research Council clinical trials unit at University College London, UK, said:
“You always go into a trial with a question to answer and an open mind, but seeing the imbalance between infections in the vaccine groups and the placebo was a surprise and one that we cannot explain. We suspect chance but cannot rule out the possibility the result is plausible, so it is clear we need to continue to support the participants and provide HIV testing to monitor the trend.”
“The total number of infections in the trial was much smaller than we expected, which is good news, and I hope this reflects what is happening in the wider community. We had seen a decline in infections from 2018 in the Registration Cohort, but even so, the placebo incidence rate was unusually low. As this impacts both vaccine results it adds to our sense of uncertainty in the comparisons.”
PrEPVacc’s Chief Investigator, Professor Pontiano Kaleebu, based at the MRC/UVRI & LSHTM Uganda Research Unit in Uganda, said:
“The vaccine questions posed in the trial have been answered. What we are clear about is that these vaccines won’t be taken any further.
“We need further immunological investigations to understand our results, and which could be used to inform future vaccine design.
“The results have been surprising, and they have been disappointing. But that is science. It has been a very good study, following the highest international standards. Now, we must advance because the world needs to have choices in its HIV prevention toolbox. A vaccine against HIV remains a critically sought and important part of that toolbox.”
Professor Jonathan Weber of Imperial College London, UK, the sponsor of PrEPVacc, said:
“Over the course of the study, it has been very good to see the number of new infections declining in all the communities we have been working in. Our study was very well-designed, involving as few participants as possible with the minimum number of infections to answer the vaccine efficacy question.
“I thank all of the hard-working staff at each site who have delivered this trial, the participants for their tireless commitment to HIV prevention, and the community members who have guided and supported us all throughout the study. I am very grateful to the national agencies and international organisations that have helped PrEPVacc throughout its journey and have given us outstanding support and guidance.”
“While it is clear that the vaccines do not produce a protective effect, we are confronted by this most unexpected result in the placebo arm, where the extremely low incidence of HIV infection is at odds with the incidence found in our Registration Cohort. We need to await the results of further experiments to understand how this surprising set of results arose, if not by chance alone.”
Xoliswa Nomvungu, a member of the Community Advisory Board at the SAMRC Verulam site, in Durban, South Africa, said:
“The study makes it clear that community engagement is imperative in all phases of research. In the PrEPVacc trial there have been positive community interactions so that people were well informed on developments, expectations, and transparency. A key take-home message from PrEPVacc is that adherence to available oral PrEP can prevent one from acquiring the HIV virus.”
Dr Peter Gilbert, Principal Investigator, HVTN Statistical and Data Management Center (SDMC), who is independent of the PrEPVacc study and has no ties with it, said:
“Given the PrEPVacc results that the estimated rates of HIV-1 acquisition were higher in the vaccine arms than the placebo arm, it is important to thoroughly quantify and communicate the precision available for drawing inferences about whether the vaccines truly elevated risk or, alternatively, a statistical fluke occurred and the vaccines were indeed safe. P-values are incomplete tools for this task because they cannot be interpreted in terms of the question, ‘What is the chance the vaccine elevated the acquisition rate?’
“To fill this gap, I conducted a Bayesian analysis that provides answers in terms of this desired interpretation, where I used the same method that I previously applied to other HIV vaccine efficacy trials. The result was that, for each vaccine, there is close to a 50-50 chance that the vaccine elevated acquisition risk vs. the vaccine was safe, as a synthesis of results over multiple ways to do the analysis, most importantly considering different prior distributions for vaccine efficacy.”
Quick facts about PrEPVacc:
PrEPVacc is an African-led, European-supported HIV prevention project that, for the first time, is combining evaluation of HIV vaccines and pre-exposure prophylaxis (PrEP) at the same time.
PrEPVacc recruited over 1,500 people aged between 18 and 40 at four trial sites in Uganda, Tanzania, and South Africa.
Preparation for the trial included a Registration Cohort (an observational study), whose first participants were enrolled in July 2018.
The first participants in the clinical trial enrolled in December 2020, and the last in March 2023. All participants had exited the trial by June 2024.
At the time of the IDMC recommendation in November 2023, PrEPVacc was the only remaining active HIV vaccine efficacy trial in the world.
A key part of the PrEPVacc project and how it is organised is to grow the capacity of African sites to do future trials themselves and foster future research leaders.
PrEPVacc is led by African researchers from Entebbe in Uganda at the MRC/UVRI and LSHTM Uganda Research Unit. They are supported by 15 partner organisations, six from Africa, six from Europe and three from the US. The Sponsor of PrEPVacc is Imperial College London. See Notes to Editors (2) for a full list of partners.
The PrEPVacc study is funded by the European & Developing Countries Clinical Trials Partnership (EDCTP) as part of the EDCTP2 Programme supported by the European Union. See Notes to Editors (3) for a full list of funders.
During the recruitment and enrolment phase, an animated video version of the participant information sheet was used to explain the study to participants. This video is at https://youtu.be/zHYC6SKKobc
PrEPVacc website: www.prepvacc.org
-ends-
Notes to Editors
(1) Oral abstract presentation at AIDS 2024: OAC08 Final vaccine efficacy results from PrEPVacc: A phase IIb HIV prophylactic vaccine trial of two active regimens each compared to placebo Eugene Ruzagira | 23 July 2024 | 16:30-17:30 | Hall B0a/Channel 4 (https://programme.aids2024.org/Programme/Session/207)
(2) PrEPVacc partners
Behind PrEPVacc there are 80 senior scientists, clinicians, social scientists, community liaison specialists and professional support roles, from 15 partner organisations. They have extensive experience working with HIV and other infectious diseases, as well as clinical trials, and specifically in carrying out HIV vaccine and PrEP trials across Europe and sub-Saharan Africa.
Medical Research Council / Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Uganda
Muhimbili University of Health and Allied Sciences, Tanzania
National Institute for Medical Research - Mbeya Medical Research Centre, Tanzania
HIV and other Infectious Diseases Research Unit, South African MRC, South Africa
Imperial College London, UK
Medical Research Council Clinical Trials Unit at University College London, UK
Centre Hospitalier Universitaire vaudois, Switzerland
Karolinska Instituet, Sweden
Medical Center of the University of Munich (LMU), Germany
International AIDS Vaccine Initiative (IAVI)
Africa Health Research Institute
EuroVacc Foundation
Gilead Sciences, Inc
Global Solutions for Infectious Diseases
East Virginia Medical School, CONRAD, USA
Military HIV Research Program at The Walter Reed Army Institute of Research (WRAIR)
https://www.prepvacc.org/partners
(3) PrEPVacc funders
PrEPVacc is a public-private partnership. The European & Developing Countries Clinical Trials Partnership (EDCTP) awarded a grant of €15M for the study and all the institutional partners are providing co-funding through staff salaries. Gilead Sciences, Inc is giving support to the project through materials, medicines, and funding. PrEPVacc is also supported by USAID and PEPFAR, USMHRP, SVRI, SAMRC, UKRI Medical Research Council, the Wellcome Trust, the Bill and Melinda Gates Foundation, SIDA, and Bundesministerium für Bildung und Forschung (BMBF).
https://www.prepvacc.org/funders
END
HIV vaccines tested in PrEPVacc fail to reduce infections
2024-07-23
ELSE PRESS RELEASES FROM THIS DATE:
Study by TU Graz shows that abrasion emissions from trains are not negligible
2024-07-23
In addition to exhaust emissions, abrasion emissions from tyres and brakes have become increasingly important when assessing the environmental impact of traffic. However, the focus here was on road vehicles; rail was hardly considered. In a study commissioned by the German Centre for Rail Transport Research (DZSF), researchers from the Institute of Thermodynamics and Sustainable Propulsion Systems at Graz University of Technology (TU Graz) have now been able to prove that so-called non-exhaust emissions from rail transport also have a relevant influence on air quality and soil pollution.
Half of the daily particulate matter limit due to trains ...
Heat-sensitive trees move uphill seeking climate change respite
2024-07-23
Trees in the Brazilian Atlantic Forest are migrating in search of more favourable temperatures with species in mountain forests moving uphill to escape rising heat caused by climate change, a new study reveals.
Most species in higher parts of the Brazilian Atlantic Forest are moving upwards as temperatures rise, but scientists say that those trees which thrive in colder temperatures are at risk of dying out as the world continues to warm.
Researchers studying the forest, which stretches along the Brazil’s Atlantic seaboard, have also discovered that some trees in ...
Arm robots are not the answer for stroke rehabilitation
2024-07-23
Commercial arm robots are increasingly deployed in order to aid stroke patients in their recovery. Around 80% of patients have problems with their arm function. Robots are also seen as a solution for financial, and staffing, shortcomings in the healthcare sector. However, research led by Amsterdam UMC now shows that they offer no clinically meaningful effects for patients. The research is published today in Neurology.
"In particular countries such as China, Japan and South Korea, but also in North America and Europe, are UL-Robots seen more ...
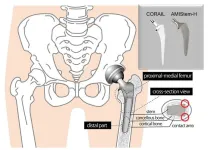
Staying hip to orthopedic advances: Comparing traditional and new hip replacement stems
2024-07-23
Osaka, Japan — Needing a hip replacement is unfortunate, but even more unfortunate is to need to do it again.
Surgeons at Osaka Metropolitan University have provided new insights into the performance of two types of stems used in total hip replacement surgery. Their findings are expected to contribute to the enhancement of long-term outcomes, improving patients’ quality of life and reducing the need for revision surgeries.
Their paper was published in The Bone & Joint Journal on June 1.
The hip joint, which connects the femur, or thighbone, to the pelvis, plays a crucial ...
Brain care score for dementia and stroke also predicts late-life depression
2024-07-23
Late-life depression, typically defined as depression with onset in individuals over 60 years of age, can affect up to a third or more of people older than 60 and can be debilitating. But, like other neurological conditions, an individual’s risk may be influenced by lifestyle choices. Researchers from Mass General Brigham previously developed and validated the Brain Care Score (BCS) for helping patients and clinicians identify lifestyle changes that may reduce their risk of dementia and stroke. Now, with collaborators at Yale University, they have shown that a higher BCS is also associated with a ...
A window of opportunity for climate change and biodiversity
2024-07-23
World leaders must take advantage of a pivotal window of opportunity for forging a much-needed joined-up approach to tackle climate change and biodiversity loss, say scientists from ZSL and York University. Without this, work on tackling either crisis could inadvertently harm progress on the other.
Published today (Tuesday 23 July) in the Journal of Applied Ecology, a paper from international conservation charity ZSL and researchers at York University, Toronto, titled ‘The Kunming-Montreal Global Biodiversity Framework and the Paris Agreement need a joint work programme for climate, nature, and people’ conceptualises how a joint work ...
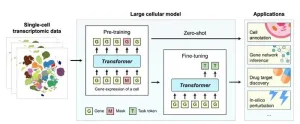
Quantitative Biology journal features groundbreaking perspectives on large cellular models
2024-07-23
In a landmark move to advance the frontiers of artificial intelligence, the Quantitative Biology (QB) journal has published a comprehensive commentary titled "Current Opinions on Large Cellular Models," highlighting the cutting-edge developments in the field of large cellular models (LCMs). The journal has brought together a consortium of leading scholars from China, the United States, and Canada to delve into the future of AI-driven biological research.
The commentary features influential authors behind some of the most impactful LCMs, such as scBERT, Geneformer, scGPT, scFoundation, and GeneCompass. These AI ...
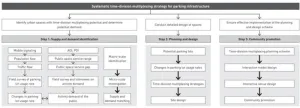
Time-division multiplexing planning and design strategies for parking lots in compact cities
2024-07-23
Compact city is an urban planning strategy aimed at promoting environmental, economic, and social sustainability through spatial configurations featured with relatively high density and mixed land use. The continuous growth in car ownership forces cities to construct more static transportation facilities such as parking lots, squeezing the activity spaces of residents and consequently giving rise to a series of efficiency and equity issues. Thus, the conflict between people and vehicles in compact cities is increasingly prominent and urgently ...
New imaging technique reveals intracellular energy dynamics in kidney cells
2024-07-23
The prevalence of kidney disease has been increasing in Japan, with it now affecting one in eight adults, but developing effective treatment remains a challenge. The kidneys are among the most energy-intensive organs in the body. For the kidneys to function, they constantly produce and consume large amounts of adenosine triphosphate (ATP), which is a chemical that the body uses to store and transport energy. However, ATP dynamics—the changes over time in ATP production and utilization—within the kidney have been poorly understood because of the lack of suitable imaging technologies.
Using a newly developed ATP imaging system, the researchers ...
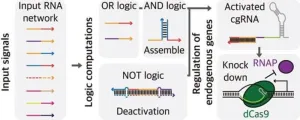
Could smart guide RNAs usher in an era of personalized medicine?
2024-07-23
Guides typically assist tourists with directions, but the experience could be greatly enhanced if they offered personalized services tailored to individual interests. Recently, researchers have transformed guide RNAs, which direct enzymes, into a smart RNA capable of controlling networks in response to various signals. This innovative research is gaining significant attention in the academic community.
A research team consisting of Professor Jongmin Kim and PhD candidates Hansol Kang and Dongwon Park from the Department of Life Sciences at POSTECH has developed a multi-signal ...