The researchers will present their results at the fall meeting of the American Chemical Society (ACS). ACS Fall 2024 is a hybrid meeting being held virtually and in person Aug. 18-22; it features about 10,000 presentations on a range of science topics.
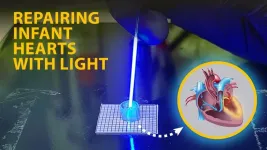
“After the surgeon first puts in the tube, these children often have to go through an additional two or three, maybe even four, surgeries just to implant a slightly larger tube,” says Christopher Rodell, who is presenting the research. “Our goal is to expand the inside of the tube with a light-emitting catheter that we insert inside the shunt, completely eliminating the need for additional surgeries.” Rodell is an assistant professor of biomedical engineering at Drexel University.
These congenital heart defects affect the organ’s lower chambers, known as ventricles, resulting in restricted blood flow to the lungs and other parts of the body. Without surgery, babies with these disorders cannot survive. Often born undersized, these infants can grow rapidly after their first shunt implantation surgery. To accommodate their growth, surgeons often perform yet another open-chest surgery. Each time this procedure is performed, it creates a risk to the child. In a study of 360 patients who underwent the initial heart reconstruction procedure, 41 needed additional surgeries to implant a larger shunt and seven died as a result.
Previously, Rodell’s colleagues at Drexel, Amy Throckmorton and Kara Spiller, built an expandable prototype to potentially replace the most commonly used type of shunt. They did so by coating the interior of the tube with a hydrogel that contains a network of water-surrounded polymers attached to each other by bonds called crosslinks. The formation of new crosslinks forces water out of the hydrogel and pulls the polymers together, contracting the hydrogel and widening the inside of the shunt. In the initial design, the new crosslinks formed automatically, without an external trigger.
Rodell joined Throckmorton and Spiller to help them reengineer the shunt so the materials would be safe for clinical use and so it could be adjusted to meet the needs of individual children. He accomplished this by developing new polymers for a hydrogel that would form new crosslinks and increase the shunt’s inner diameter in response to a trigger. To initiate crosslinking on demand, Rodell decided to use blue light because this wavelength carries enough energy to initiate the reaction but is safe for living tissue.
“Light has always been one of my favorite triggers, because you can control when and where you apply it,” Rodell says.
For the new device, Rodell and his team led by graduate student Akari Seiner are using a fiber-optic catheter, essentially a long, thin tube with a light-emitting tip. To activate the light-sensitive hydrogel inside the shunt, they intend for surgeons to insert the catheter into an artery near the armpit then maneuver it into position, eliminating the need to open the baby’s chest.
In lab experiments, they found they could expand the shunt incrementally, with the amount of expansion varying according to the length of light exposure — results indicating that once implanted, adjustments to the shunt could be customized to each child. They found they could dilate the shunt by as much as 40%, expanding its diameter from 3.5 millimeters to 5 millimeters — nearly the size of the largest shunt implanted in children. They also assessed how blood cells and blood vessels might respond to the modified shunt. They found no evidence that the implanted tube caused the formation of blood clots, an inflammatory response or other reactions that could pose potential health problems.
Next, the team plans to test full-length shunt prototypes in an artificial set-up mimicking the human circulatory system. If these experiments are successful, the researchers intend to move on to experiments in animal models. This technology could be useful beyond single-ventricle heart disorders, according to Rodell. Surgeons could, for example, use similar tubes to replace blood vessels in children injured in a car accident.
“In these procedures, you run into the same problem: Children aren’t just tiny adults; they continue to grow,” Rodell says. “That’s something we need to account for in biomaterials, how that graft will behave over time.”
The research was funded by The Hartwell Foundation.
A Headline Science video about this topic will be posted on Monday, Aug. 19. Reporters can access the video during the embargo period, and once the embargo is lifted the same URL will allow the public to access the content. Visit the ACS Fall 2024 program to learn more about this presentation, “Development of a geometrically-tunable blood shunt for pediatric heart reconstruction surgery,” and other science presentations.
###
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
Registered journalists can subscribe to the ACS journalist news portal on EurekAlert! to access embargoed and public science press releases. For media inquiries, contact newsroom@acs.org.
Note to journalists: Please report that this research was presented at a meeting of the American Chemical Society. ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Follow us: X, formerly Twitter | Facebook | LinkedIn | Instagram
Title
Development of a geometrically-tunable blood shunt for pediatric heart reconstruction surgery
Abstract
Single-ventricle malformations are severe congenital heart defects that require life-saving reconstructive surgery, placing a blood shunt (fixed-diameter tubing) to divert blood flow to the lungs. Mortality rates are the highest in all cardiothoracic surgery. This is because shunts are unable to grow with the child, necessitating repeated surgeries to implant larger shunts. Here, we develop a light-responsive shunt that changes in diameter on demand, allowing blood flow to be tuned to meet the child’s growing needs without surgery.
Dextran was methacrylated by glycidyl methacrylate (50% substitution) and crosslinked into hydrogels (10%w/v) by Michael-addition with dithiothreitol (DTT; DTT/methacrylate ratios of 20-50%). Hydrogel volumes and moduli were assessed at baseline and after secondary photopolymerization performed to induce de-swelling. 40% DTT/methacrylate afforded excellent volume reduction (40%) with moduli (8.46±0.67kPa) comparable to soft connective tissues and was selected for prototype development. Hydrogels did not hinder cell proliferation (PrestoBlue), induce inflammatory response (RAW-Blue reporter assay) or hemolysis (ASTM 756-13). PTFE tubing was surface modified by polydopamine to enable hydrogel adhesion. When irradiated via a fiberoptic catheter, hydrogels contracted towards the outer tubing. Clinically-indicated increases in lumen diameter (15-18%) necessary to support infant growth were obtained in <1min; changes far-exceeding clinical demands (40%) were possible. The dual-stage crosslinking approach is a promising strategy to create responsive biomedical implants that change in size, and the geometrically-tunable shunt design may prevent repeated surgeries in this fragile patient population.
END