A team of scientists studying cell division developed a special light microscopy system and used it to analyze the molecular density of cellular environments. Their results provide a novel insight into mitotic chromosome condensation in living human cells.
Their work is published in the journal Proceedings of the National Academy of Sciences (PNAS) on August 27, 2024.
DOI:https://doi.org/10.1073/pnas.2403153121
To carry out their study, the team developed an orientation-independent-differential interference contrast (OI-DIC) microscopy system combined with a confocal laser scanning microscope capable of precisely mapping optical path differences and estimating molecular densities.
“Mitotic chromosome condensation is an essential process for transmitting replicated chromosomes to two daughter cells during cell division. To study the underlying physical principles of this process, we investigated whether depletion attraction, a force that attracts large structures in crowded cell environments, is involved in this process,” said Shiori Iida, from the Genome Dynamics Laboratory, National Institute of Genetics and the Graduate Institute for Advanced Studies, SOKENDAI.
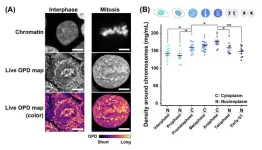
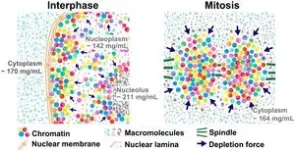
In the chromosome condensation process, chromatin strands are compacted into short chromosomes. This process makes the chromosomes rigid to resist the pulling force from the spindle. While several proteins involved in the condensation process, including condensins and topoisomerase IIα, have been identified and extensively investigated, physical bases of the condensation process remain unclear. “Using newly developed special light microscopy, which can image the molecular density of cellular environments, we found that crowding around chromosomes increases during cell division (Figure 1), leading to a rise in depletion attraction. Our results suggest that the rise in depletion attraction renders chromosomes more rigid, ensuring accurate chromosome transmission during cell division (Figure 2),” said Iida.
“Our novel light microscopy system allowed us to obtain matched high-resolution optical path difference and confocal images to get precise absolute densities in live cells. This OI-DIC imaging is capable of generating a 3D volume image of the refractive index and molecular density, or dry mass, in living cells. With the OI-DIC system, we quantified the absolute density of the molecules around chromosomes during mitosis of living cells,” said Michael Shribak from the Marine Biological Laboratory, Woods Hole. The team analyzed the absolute densities in cytoplasm and nucleoplasm in various cell cycle stages of human HCT116 cells and Indian Muntjac DM cells. The human HCT116 cells are human colon cancer cells. The Indian Muntjac are deer that have the lowest chromosome count of any mammal, making their cells ideal for mitosis research.
Their analysis showed that the molecular density surrounding chromosomes increased with the progression from the prophase to anaphase stages of mitosis, concurring with chromosome condensation (Figure 1). However, the molecular density went down in telophase, when chromosome decondensation began. Using hypotonic or hypertonic treatment of the mitotic cells, the team observed consistent changes in the condensation levels of chromosomes. With hypertonic treatment of the mitotic cells, the density and induced chromosome condensation rose rapidly, while hypotonic treatment had the opposite effect.
Then to further support their findings in HCT116 cells, the team examined another cell line, the Indian Muntjac DM cells. These DM cells were derived from deer fibroblast cells and have very large mitotic chromosomes. The results they obtained with the DM cells supported their findings with the human HCT116 cells. This suggests that a transient rise in the density of chromosome environment may be a common feature in mitotic cells.
The team discovered that the higher concentrations of macromolecules condense chromatin and make it stiffer and more solid-like in vitro. “To our knowledge, we are the first to demonstrate that the depletion attraction/macromolecular crowding converts fibrous chromatin condensates formed by cations into liquid droplets in vitro,” said Kazuhiro Maeshima from the Genome Dynamics Laboratory, National Institute of Genetics and the Graduate Institute for Advanced Studies, SOKENDAI.
“Our results suggest that the rise in depletion attraction renders chromosomes more rigid, ensuring accurate chromosome transmission during cell division. A transient rise in the molecular density appears to be induced by nuclear membrane breakdown during mitosis (Figure 2). Upon nuclear membrane breakdown, the nuclear envelope, nuclear pore complexes, nuclear lamina, and nucleoli are disassembled into small pieces. As a result, cytoplasmic and nucleolar factors are exposed to the chromosomes and fully contribute to an increase in depletion attraction. This provides us with a novel insight into the physical bases of mitotic chromosome condensation in living human cells,” said Maeshima.
“In this study, we discovered that mitotic chromosomes are condensed by a physical force known as depletion attraction during cell division. Looking ahead, we aim to elucidate how physical properties of DNA, and the physical forces affecting their properties contribute to DNA transactions, including transcription, DNA replication and repair,” said Iida.
The research team includes: Shiori Iida, Satoru Ide, and Kazuhiro Maeshima from the Genome Dynamics Laboratory, National Institute of Genetics and the Graduate Institute for Advanced Studies, SOKENDAI; Sachiko Tamura from the Genome Dynamics Laboratory, National Institute of Genetics; Masaki Sasai from the Fukui Institute for Fundamental Chemistry, Kyoto University and the Department of Complex Systems Science, Nagoya University; Tomomi Tani from the Biomedical Research Institute, National Institute of Advanced Industrial Science and Technology; Tatsuhiko Goto from the Research Center for Global Agromedicine and Department of Life and Food Sciences, Obihiro University of Agriculture and Veterinary Medicine; and Michael Shribak from the Marine Biological Laboratory, Woods Hole.
The research is funded by grants from the Japan Society for the Promotion of Science, Takeda Science Foundation, Japan Science and Technology Agency, Inoué Endowment Fund, and NIGMS/NIH.
###
About National Institute of Genetics (NIG)
National Institute of Genetics (NIG) was established to carry out broad and comprehensive research in genetics. NIG contributes to the development of academic research as one of the inter-university research institutes constituting the Research Organization of Information and Systems (ROIS).
About the Research Organization of Information and Systems (ROIS)
ROIS is a parent organization of four national institutes (National Institute of Polar Research, National Institute of Informatics, the Institute of Statistical Mathematics and National Institute of Genetics) and the Joint Support-Center for Data Science Research. It is ROIS's mission to promote integrated, cutting-edge research that goes beyond the barriers of these institutions, in addition to facilitating their research activities, as members of inter-university research institutes.
END