Parkinson’s disease (PD), the second most common neurodegenerative disorder globally, has long baffled scientists with its progressive nature and debilitating effects on motor function.
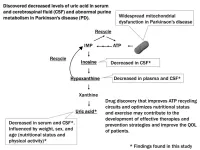
A recent study from the School of Medicine at Fujita Health University has brought new insights into the metabolic disruptions experienced by patients with PD. By analyzing the blood and cerebrospinal fluid (CSF) of the patients, researchers have discovered critical impairments in purine metabolism and the recycling of adenosine triphosphate (ATP)—the molecule responsible for energy production in cells.
For years, scientists have noted the decreased levels of uric acid in patients with PD. Uric acid, a compound known for its antioxidant properties, was initially thought to play a direct role in the disease by reducing oxidative stress in the brain. However, this study, published in NPJ Parkinson’s Disease on 09 September 2024 reveals that the situation is more complex.
“Our findings suggest that decreased uric acid levels in patients with PD are influenced by factors beyond purine metabolism, including external factors such as sex, weight, and age,” said Dr. Watanabe, the lead author of the study. “This means the relationship between uric acid and PD is more nuanced than we previously thought, and it's about more than just oxidative stress.”
By assessing the levels of purine metabolites, including inosine, hypoxanthine, xanthine, and uric acid, using a technique called targeted metabolomics, they found that patients with PD have significantly lower levels of uric acid in both serum and CSF compared to healthy controls. Additionally, the levels of hypoxanthine, another purine metabolite, were reduced.
According to the study, the reductions in serum and CSF uric acid were linked to body weight and sex, but not to the upstream metabolite xanthine, which challenges prior assumptions about purine metabolism. As Dr. Watanabe explains, “Our findings indicate that serum and CSF uric acid levels are not directly related to upstream xanthine concentrations, suggesting influences beyond traditional purine metabolism pathways.”
This discovery is crucial, as it points to an impairment in the ATP recycling system. ATP is essential for cellular energy use, and its breakdown and recycling are vital for maintaining healthy cell function. In PD, this system seems to be malfunctioning, leading to an energy deficit that could further exacerbate the disease’s symptoms.
Another important aspect of the study involved inosine, a precursor to uric acid. The team observed a significant reduction in CSF inosine in patients with PD, but no significant drop in serum inosine. As Dr. Watanabe explains, “Our study found that the reduction in CSF inosine may reflect a decrease in nucleotide production within the central nervous system, and this could have major implications for energy production in the brain.”
The study notably found that, compared with healthy controls, patients with PD had substantially lower serum and CSF hypoxanthine levels. Over 90% of hypoxanthine is recycled as inosine monophosphate (IMP) in the salvage pathway, which plays a crucial role in maintaining energy production.
The discovery that energy metabolism is disrupted in PD opens the door to new treatment strategies. Current therapies largely focus on managing symptoms, but this research suggests that targeting the body’s energy recycling system could slow the disease’s progression. This study shows that treatments aimed at elevating serum uric acid levels might have limited efficacy in improving Parkinsonism. Instead, focusing on the purine recycling system, particularly enhancing ATP production, could hold more promise.
As the research progresses, the team hopes to explore exercise and nutritional interventions as possible ways to improve energy metabolism and ATP recycling.
In conclusion, this incredible research brings us one step closer to understanding the complex metabolic changes involved in PD. By focusing on energy metabolism and the purine recycling system, scientists could develop new treatments that not only slow the progression of the disease but also improve the quality of life for patients.
***
Reference
DOI: https://doi.org/10.1038/s41531-024-00785-0
About Fujita Health University
Fujita Health University is a private university situated in Toyoake, Aichi, Japan. It was founded in 1964 and houses one of the largest teaching university hospitals in Japan in terms of the number of beds. With over 900 faculty members, the university is committed to providing various academic opportunities to students internationally. Fujita Health University has been ranked eighth among all universities and second among all private universities in Japan in the 2020 Times Higher Education (THE) World University Rankings. THE University Impact Rankings 2019 visualized university initiatives for sustainable development goals (SDGs). For the “good health and well-being” SDG, Fujita Health University was ranked second among all universities and number one among private universities in Japan. The university became the first Japanese university to host the "THE Asia Universities Summit" in June 2021. The university’s founding philosophy is “Our creativity for the people (DOKUSOU-ICHIRI),” which reflects the belief that, as with the university’s alumni and alumnae, current students also unlock their future by leveraging their creativity.
Website: https://www.fujita-hu.ac.jp/en/index.html
About Professor Prof. Hirohisa Watanabe from Fujita Health University
Prof. Hirohisa Watanabe received his Ph.D. from Nagoya University. He is currently a Professor at the Institute of the Department of Neurology, Fujita Health University. He worked as a Designated professor at the Brain and Mind Research Center Division, Nagoya University (2013–2019). His research interests and expertise include aging, neuroradiology, biomarkers, Parkinson's disease, brain networks, and brain energy.
Funding information
This work was supported by JSPS KAKENHI Grant Number JP22K07508.
END