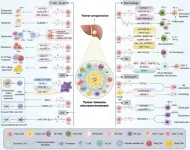
The TIME is a dynamic network composed of cancer cells, immune cells, and stromal components. During the early stages of tumorigenesis, the TIME attempts to eliminate abnormal cells through immune surveillance. However, as tumors evolve, they exploit the TIME to evade immune responses and promote tumor progression. Exosomes play a central role in this transformation by transporting bioactive molecules such as proteins, lipids, and nucleic acids, thereby influencing processes like immune escape, angiogenesis, and metastasis.
HCC is particularly adept at manipulating the TIME via exosome-mediated mechanisms. These vesicles reprogram immune and stromal cells, creating an environment conducive to tumor growth. Understanding these mechanisms is critical for developing novel therapeutic strategies.
Exosome Biogenesis and Function
Exosome formation is a multistep process that begins with the inward budding of the plasma membrane to form early endosomes. These endosomes mature into multivesicular bodies (MVBs), which either fuse with lysosomes for degradation or with the plasma membrane to release their intraluminal vesicles as exosomes. Key regulators of this process include the Rab family of GTPases, SNARE proteins, and the endosomal sorting complex required for transport (ESCRT).
Exosomes carry diverse cargo, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), and proteins, enabling them to modulate recipient cell behavior. For instance, HCC-derived exosomes have been shown to enhance immune evasion, promote angiogenesis, and drive metastasis by altering the TIME. Their role in transporting immune checkpoint molecules such as PD-L1 further underscores their impact on tumor immune evasion.
HCC-Derived Exosomes in Immune Modulation
HCC-derived exosomes significantly influence various immune cells within the TIME:
Innate Immune Cells:
Macrophages: HCC exosomes polarize tumor-associated macrophages (TAMs) towards the pro-tumorigenic M2 phenotype. For example, miRNAs such as miR-452-5p and lncRNA HMMR-AS1 within these exosomes modulate signaling pathways that suppress anti-tumor responses. Exosomal miR-142-3p from hepatitis B virus (HBV)-infected HCC cells induces ferroptosis in M1 macrophages, further undermining immune surveillance.
Dendritic Cells (DCs): While exosomes can prime DCs to activate T cells, they also inhibit DC maturation and antigen presentation in advanced stages of HCC, contributing to immune suppression.
Natural Killer (NK) Cells: Exosomes carrying molecules like circUHRF1 induce NK cell exhaustion by upregulating inhibitory receptors, reducing their cytotoxic potential.
Adaptive Immune Cells:
CD8+ T Cells: Exosomes transfer immune checkpoint molecules like PD-L1 and regulatory RNAs such as circCCAR1 to CD8+ T cells, leading to dysfunction and exhaustion. This results in diminished anti-tumor immunity and resistance to therapies like PD-1 inhibitors.
CD4+ T Cells: Exosomes promote the differentiation of immunosuppressive regulatory T cells (Tregs) while disrupting the balance of T helper (Th) subsets. Exosomal circGSE1, for instance, drives Treg proliferation, facilitating immune evasion.
B Cells: HCC-derived exosomes enhance the immunosuppressive functions of regulatory B cells (Bregs), contributing to a tumor-promoting microenvironment.
Non-HCC-Derived Exosomes and Crosstalk in the TIME
Exosomes from non-HCC sources also modulate the TIME:
M1 Macrophage-Derived Exosomes: These vesicles exhibit anti-tumor properties by delivering miRNAs that inhibit oncogenic pathways in HCC cells.
Cancer-Associated Fibroblast (CAF)-Derived Exosomes: These vesicles promote stromal remodeling and immune suppression, enhancing tumor progression.
Potential Therapeutic Applications
The unique properties of exosomes, such as their biocompatibility and targeted delivery capabilities, make them attractive for therapeutic applications. Exosome-based immunotherapies aim to reverse immune suppression and re-activate anti-tumor responses. For instance, engineering exosomes to carry tumor antigens or immune-activating molecules has shown promise in preclinical models. However, challenges such as off-target effects and the complexity of exosome isolation and manipulation remain.
Future Directions
To harness the therapeutic potential of exosomes, further research is needed to elucidate their molecular mechanisms within the TIME. Advances in exosome engineering could lead to the development of targeted therapies that enhance the efficacy of existing treatments, such as immune checkpoint inhibitors.
Conclusions
This review highlights the pivotal role of exosomes in shaping the TIME and driving HCC progression. By advancing our understanding of their regulatory mechanisms, we can unlock new avenues for innovative therapeutic strategies, offering hope for improved outcomes in this challenging disease.
Full text
https://www.xiahepublishing.com/2310-8819/JCTH-2024-00302
The study was recently published in the Journal of Clinical and Translational Hepatology.
The Journal of Clinical and Translational Hepatology (JCTH) is owned by the Second Affiliated Hospital of Chongqing Medical University and published by XIA & HE Publishing Inc. JCTH publishes high quality, peer reviewed studies in the translational and clinical human health sciences of liver diseases. JCTH has established high standards for publication of original research, which are characterized by a study’s novelty, quality, and ethical conduct in the scientific process as well as in the communication of the research findings. Each issue includes articles by leading authorities on topics in hepatology that are germane to the most current challenges in the field. Special features include reports on the latest advances in drug development and technology that are relevant to liver diseases. Regular features of JCTH also include editorials, correspondences and invited commentaries on rapidly progressing areas in hepatology. All articles published by JCTH, both solicited and unsolicited, must pass our rigorous peer review process.
Follow us on X: @xiahepublishing
Follow us on LinkedIn: Xia & He Publishing Inc.
END