(Press-News.org) In the future, delivering therapeutic drugs exactly where they are needed within the body could be the task of miniature robots. Not little metal humanoid or even bio-mimicking robots; think instead of tiny bubble-like spheres.
Such robots would have a long and challenging list of requirements. For example, they would need to survive in bodily fluids, such as stomach acids, and be controllable, so they could be directed precisely to targeted sites. They also must release their medical cargo only when they reach their target, and then be absorbable by the body without causing harm.
Now, microrobots that tick all those boxes have been developed by a Caltech-led team. Using the bots, the team successfully delivered therapeutics that decreased the size of bladder tumors in mice. A paper describing the work appears in the journal Science Robotics.
"We have designed a single platform that can address all of these problems," says Wei Gao, professor of medical engineering at Caltech, Heritage Medical Research Institute Investigator, and co-corresponding author of the new paper about the bots, which the team calls bioresorbable acoustic microrobots (BAM).
"Rather than putting a drug into the body and letting it diffuse everywhere, now we can guide our microrobots directly to a tumor site and release the drug in a controlled and efficient way," Gao says.
The concept of micro- or nanorobots is not new. People have been developing versions of these over the past two decades. However, thus far, their applications in living systems have been limited because it is extremely challenging to move objects with precision in complex biofluids such as blood, urine, or saliva, Gao says. The robots also have to be biocompatible and bioresorbable, meaning that they leave nothing toxic behind in the body.
The Caltech-developed microrobots are spherical microstructures made of a hydrogel called poly(ethylene glycol) diacrylate. Hydrogels are materials that start out in liquid or resin form and become solid when the network of polymers found within them becomes cross-linked, or hardens. This structure and composition enable hydrogels to retain large amounts of fluid, making many of them biocompatible. The additive manufacturing fabrication method also enables the outer sphere to carry the therapeutic cargo to a target site within the body.
To develop the hydrogel recipe and to make the microstructures, Gao turned to Caltech's Julia R. Greer, the Ruben F. and Donna Mettler Professor of Materials Science, Mechanics and Medical Engineering, the Fletcher Jones Foundation Director of the Kavli Nanoscience Institute, and co-corresponding author of the paper. Greer's group has expertise in two-photon polymerization (TPP) lithography, a technique that uses extremely fast pulses of infrared laser light to selectively cross-link photosensitive polymers according to a particular pattern in a very precise manner. The technique allows a structure to be built up layer by layer, in a way reminiscent of 3D printers, but in this case, with much greater precision and form complexity.
Greer’s group managed to "write," or print out, microstructures that are roughly 30 microns in diameter—about the diameter of a human hair.
"This particular shape, this sphere, is very complicated to write," Greer says. "You have to know certain tricks of the trade to keep the spheres from collapsing on themselves. We were able to not only synthesize the resin that contains all the biofunctionalization and all the medically necessary elements, but we were able to write them in a precise spherical shape with the necessary cavity."
In their final form, the microrobots incorporate magnetic nanoparticles and the therapeutic drug within the outer structure of the spheres. The magnetic nanoparticles allow the scientists to direct the robots to a desired location using an external magnetic field. When the robots reach their target, they remain in that spot, and the drug passively diffuses out.
Gao and colleagues designed the exterior of the microstructure to be hydrophilic—that is, attracted to water—which ensures that the individual robots do not clump together as they travel through the body. However, the inner surface of the microrobot cannot be hydrophilic because it needs to trap an air bubble, and bubbles are easy to collapse or dissolve.
To construct hybrid microrobots that are both hydrophilic on their exterior and hydrophobic, or repellent to water, in their interior, the researchers devised a two-step chemical modification. First, they attached long-chain carbon molecules to the hydrogel, making the entire structure hydrophobic. Then the researchers used a technique called oxygen plasma etching to remove some of those long-chain carbon structures from the interior, leaving the outside hydrophobic and the interior hydrophilic.
"This was one of the key innovations of this project," says Gao, who is also a Ronald and JoAnne Willens Scholar. "This asymmetric surface modification, where the inside is hydrophobic and the outside is hydrophilic, really allows us to use many robots and still trap bubbles for a prolonged period of time in biofluids, such as urine or serum."
Indeed, the team showed that the bubbles can last for as long as several days with this treatment versus the few minutes that would otherwise be possible.
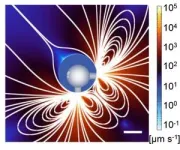
The presence of trapped bubbles is also crucial for moving the robots and for keeping track of them with real-time imaging. For example, to enable propulsion, the team designed the microrobot sphere to have two cylinder-like openings—one at the top and another to one side. When the robots are exposed to an ultrasound field, the bubbles vibrate, causing the surrounding fluid to stream away from the robots through the opening, propelling the robots through the fluid. Gao's team found that the use of two openings gave the robots the ability to move not only in various viscous biofluids, but also at greater speeds than can be achieved with a single opening.
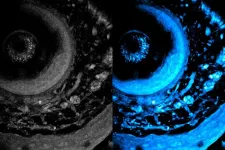
Trapped within each microstructure is an egg-like bubble that serves as an excellent ultrasound imaging contrast agent, enabling real-time monitoring of the bots in vivo. The team developed a way to track the microrobots as they move to their targets with the help of ultrasound imaging experts Mikhail Shapiro, Caltech's Max Delbruck Professor of Chemical Engineering and Medical Engineering, a Howard Hughes Medical Institute Investigator; co-corresponding author Di Wu, research scientist and director of the DeepMIC Center at Caltech; and co-corresponding author Qifa Zhou, professor of ophthalmology and biomedical engineering at USC.
The final stage of development involved testing the microrobots as a drug-delivery tool in mice with bladder tumors. The researchers found that four deliveries of therapeutics provided by the microrobots over the course of 21 days was more effective at shrinking tumors than a therapeutic not delivered by robots.
"We think this is a very promising platform for drug delivery and precision surgery," Gao says. "Looking to the future, we could evaluate using this robot as a platform to deliver different types of therapeutic payloads or agents for different conditions. And in the long term, we hope to test this in humans."
The lead authors of the paper, "Imaging-guided bioresorbable acoustic hydrogel microrobots," are Hong Han (MS ’23) and Xiaotian Ma (MS ’24) from Gao’s lab, Weiting Deng (PhD ’24), now a post-doc at UCLA who conducted this work while in Greer's lab, and Junhang Zhang from Zhou's lab at USC. Additional Caltech authors are Songsong Tang, Ernesto Criado-Hidalgo, Emil Karshalev (now at General Atomics), Jounghyun Yoo, Ming You, Ann Liu, Canran Wang (MS ’23), Hao K. Shen, Payal N. Patel, Claire L. Hays, Peter J. Gunnarson (PhD ’24), Lei Li (PhD ’19), Yang Zhang, John O. Dabiri (PhD ’05), Caltech’s Centennial Professor of Aeronautics and Mechanical Engineering; and Lihong V. Wang, Caltech’s Bren Professor of Medical Engineering and Electrical Engineering, and the Andrew and Peggy Cherng Medical Engineering Leadership Chair. Additional authors are On Shun Pak of Santa Clara University, Lailai Zhu of National University of Singapore, and Chen Gong of USC.
The work was supported by the Kavli Nanoscience Institute at Caltech as well as by funding from the National Science Foundation; the Heritage Medical Research Institute; the Singapore Ministry of Education Academic Research Fund; the National Institutes of Health; the Army Research Office through the Institute for Collaborative Biotechnologies; the Caltech DeepMIC Center, with support of the Caltech Beckman Institute and the Arnold and Mabel Beckman Foundation; and the David and Lucile Packard Foundation.
END
Caltech creates minuscule robots for targeted drug delivery
2024-12-11
ELSE PRESS RELEASES FROM THIS DATE:
Noninvasive imaging method can penetrate deeper into living tissue
2024-12-11
Metabolic imaging is a noninvasive method that enables clinicians and scientists to study living cells using laser light, which can help them assess disease progression and treatment responses.
But light scatters when it shines into biological tissue, limiting how deep it can penetrate and hampering the resolution of captured images.
Now, MIT researchers have developed a new technique that more than doubles the usual depth limit of metabolic imaging. Their method also boosts imaging speeds, yielding richer and more detailed images.
This new technique does not require tissue to be ...
Researchers discover zip code that allows proteins to hitch a ride around the body
2024-12-11
Researchers at The Ottawa Hospital and the University of Ottawa have discovered an 18-digit code that allows proteins to attach themselves to exosomes - tiny pinched-off pieces of cells that travel around the body and deliver biochemical signals. The discovery, published in Science Advances, has major implications for the burgeoning field of exosome therapy, which seeks to harness exosomes to deliver drugs for various diseases.
“Proteins are the body’s own home-made drugs, but they don’t necessarily travel well around the body,” said Dr. Michael Rudnicki, senior ...
The distinct nerve wiring of human memory
2024-12-11
The black box of the human brain is starting to open. Although animal models are instrumental in shaping our understanding of the mammalian brain, scarce human data is uncovering important specificities. In a paper published in Cell, a team led by the Jonas group at the Institute of Science and Technology Austria (ISTA) and neurosurgeons from the Medical University of Vienna shed light on the human hippocampal CA3 region, central for memory storage.
Many of us have relished those stolen moments with a grandparent by the fireplace, our hearts racing to the intrigues of their stories from good old times, recounted with vivid imagery ...
Researchers discover new third class of magnetism that could transform digital devices
2024-12-11
A new class of magnetism called altermagnetism has been imaged for the first time in a new study. The findings could lead to the development of new magnetic memory devices with the potential to increase operation speeds of up to a thousand times.
Altermagnetism is a distinct form of magnetic order where the tiny constituent magnetic building blocks align antiparallel to their neighbours but the structure hosting each one is rotated compared to its neighbours.
Scientists from the University of Nottingham’s School of Physics and Astonomy have shown that this new third class ...
Personalized blood count could lead to early intervention for common diseases
2024-12-11
A complete blood count (CBC) screening is a routine exam requested by most physicians for healthy adults. This clinical test is a valuable tool for assessing a patient’s overall health from one blood sample. Currently, the results of CBC tests are analyzed using a one-size-fits-all reference interval, but a new study led by researchers from Mass General Brigham suggests that this approach can lead to overlooked deviations in health. In a retrospective analysis, researchers show that these reference intervals, or setpoints, are unique to each patient. The study revealed that one healthy ...
Innovative tissue engineering: Boston University's ESCAPE method explained
2024-12-11
When it comes to the human body, form and function work together. The shape and structure of our hands enable us to hold and manipulate things. Tiny air sacs in our lungs called alveoli allow for air exchange and help us breath in and out. And tree-like blood vessels branch throughout our body, delivering oxygen from our head to our toes. The organization of these natural structures holds the key to our health and the way we function. Better understanding and replicating their designs could help us unlock biological insights for more effective drug-testing, and the development of new therapeutics and organ replacements. Yet, biologically engineering tissue ...
Global healthspan-lifespan gaps among 183 WHO member states
2024-12-11
About The Study: This study identifies growing healthspan (years lived in good health)-lifespan gaps around the globe, threatening healthy longevity across worldwide populations. Women globally exhibited a larger healthspan-lifespan gap than men.
Corresponding Author: To contact the corresponding author, Andre Terzic, MD, PhD, email terzic.andre@mayo.edu.
To access the embargoed study: Visit our For The Media website at this link https://media.jamanetwork.com/
(doi:10.1001/jamanetworkopen.2024.50241)
Editor’s Note: Please see the article ...
Stanford scientists transform ubiquitous skin bacterium into a topical vaccine
2024-12-11
Imagine a world in which a vaccine is a cream you rub onto your skin instead of a needle a health care worker pushes into your one of your muscles. Even better, it’s entirely pain-free and not followed by fever, swelling, redness or a sore arm. No standing in a long line to get it. Plus, it’s cheap.
Thanks to Stanford University researchers’ domestication of a bacterial species that hangs out on the skin of close to everyone on Earth, that vision could become a reality.
“We all hate needles — everybody does,” said Michael Fischbach, PhD, the Liu (Liao) Family Professor and a professor of bioengineering. “I haven’t found a single person ...
Biological diversity is not just the result of genes
2024-12-11
How can we explain the morphological diversity of living organisms? Although genetics is the answer that typically springs to mind, it is not the only explanation. By combining observations of embryonic development, advanced microscopy, and cutting-edge computer modelling, a multi-disciplinary team from the University of Geneva (UNIGE) demonstrate that the crocodile head scales emerge from the mechanics of growing tissues, rather than molecular genetics. The diversity of these head scales observed in different crocodilian species therefore arises from the evolution of mechanical parameters, such as the growth ...
Analysis reveals an additional mechanism behind statin therapy’s heart-related benefits in people with HIV
2024-12-11
Investigators who previously found that a daily statin pill helps prevent heart attacks and strokes in people with HIV have now discovered a potential mechanism that may help to stabilize plaques and prevent their rupture in blood vessels. The research led by a team from Mass General Brigham is published in JAMA Cardiology.
“Individuals with HIV tend to have an excess of noncalcified plaques that are vulnerable to rupture at a younger age, putting them at high risk for strokes, heart attacks, and sudden cardiac death,” said senior author Steven Grinspoon, MD, chief of the Metabolism Unit at Massachusetts General Hospital, a founding member of the Mass General Brigham healthcare ...