(Press-News.org) The Alliance for Clinical Trials in Oncology today announced the results of a data analysis from a randomized phase III clinical trial involving patients with stage III colon cancer, which found that adding the drug celecoxib to treatment after surgery might help those who still have traces of cancer in their blood. The analysis showed that patients with signs of cancer in their blood measured by Signatera™, a circulating tumor DNA (ctDNA) test, tended to have worse outcomes. However, those who took celecoxib after surgery had a much better chance of staying cancer-free. These results are being presented in a late-breaking abstract and highlighted in the press program during the 2025 American Society of Clinical Oncology Gastrointestinal Cancers (ASCO GI) Symposium, in San Francisco, CA.
"These findings highlight the critical role of ctDNA status in predicting cancer recurrence and chemotherapy benefit for patients with stage III colon cancer,” said Jonathan Nowak, MD, PhD, lead author and molecular and gastrointestinal pathologist at Dana-Farber Cancer Institute and Brigham and Women's Hospital. “For those with detectable traces of cancer in their blood after surgery, adding celecoxib to their treatment regimen may significantly improve disease-free survival, offering a new opportunity to enhance outcomes for this high-risk group."
The CALGB (Alliance)/SWOG 80702 trial assessed the benefit of adding celecoxib, a non-steroidal anti-inflammatory drug (NSAID), to FOLFOX chemotherapy in the postoperative treatment of stage III colorectal cancer (CRC) without selecting patients based on specific biomarkers. The trial also compared three-month versus six-month FOLFOX treatment as part of the larger International Duration Evaluation of Adjuvant Therapy (IDEA) collaboration. The original prospective trial enrolled 2,526 patients between 2010 and 2015. While the addition of celecoxib did not significantly improve disease-free survival (results were published in 2021), NSAIDs have shown potential benefits in certain CRC subgroups, such as reducing the risk of developing precancerous colon polyps.
According to Jeffrey Meyerhardt, MD, MPH, senior author and co-director of the Colon and Rectal Cancer Center at Dana-Farber Cancer Institute, in the original prospective celecoxib clinical trial designed 10 years ago, patients were evaluated before and after surgery using imaging, which can show where cancer cells have gathered but has limited ability to detect smaller or microscopic clusters. Current ctDNA tests offer a more sensitive way to detect whether cancer remains after surgery by identifying tiny fragments of tumor DNA in the blood.
The subsequent data analysis to be presented at ASCO GI today includes 1,011 of 2,526 CRC patients with available post-surgery biobanked samples, and investigates the ability of Signatera to identify a subgroup of patients who may benefit from adding celecoxib to FOLFOX, as well as others who may not require six months of FOLFOX. Disease free-survival and overall survival are the study’s primary and secondary endpoints, respectively.
Researchers performed ctDNA tests on blood samples taken after surgery. Their analysis revealed that those with positive ctDNA generally had worse outcomes. However, those with positive ctDNA tests who had been prescribed celecoxib with standard chemotherapy had significantly improved disease-free survival compared to those who took a placebo. For those who had negative ctDNA tests, there was no significant difference between those taking celecoxib versus placebo.
Both researchers state, "The analysis suggests that celecoxib with standard chemotherapy shows significant promise for patients with early-stage colon cancer who have residual disease after initial treatment. This evidence, along with findings from other ongoing studies, will help identify which patients might benefit from adding celecoxib to their standard treatment regimen."
# # #
Reference: CALGB (Alliance)/SWOG 80702: A phase III trial of 6 versus 12 treatments of adjuvant FOLFOX plus celecoxib or placebo for patients with resected stage III colon cancer. A full description of this clinical trial can be found at https://clinicaltrials.gov/study/NCT01150045
The Alliance for Clinical Trials in Oncology develops and conducts clinical trials with promising new cancer therapies, and utilizes the best science to develop optimal treatment and prevention strategies for cancer, as well as research methods to alleviate side effects of cancer and cancer treatments. The Alliance is part of the National Clinical Trials Network (NCTN) funded by the National Cancer Institute (NCI) and serves as a research base for the NCI Community Research Oncology Program (NCORP). The Alliance comprises nearly 10,000 cancer specialists at hospitals, medical centers, and community clinics across the United States and Canada. Chair: Evanthia Galanis, MD. To learn more, visit www.AllianceforClinicalTrialsinOncology.org.
END
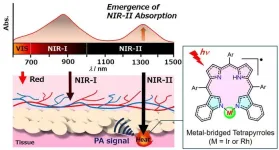
Tokyo, Japan – Researchers from Tokyo Metropolitan University have developed a new dye that can strongly absorb second near-IR radiation and transform it to heat. Starting with a dye from the bile pigment family, they designed a unique ring structure which can bind rhodium and iridium. Measurements and modeling revealed strong second near-IR absorptions and exceptional photostability. Second near-IR waves easily penetrate human tissue; the new dye may be applied in deep tissue therapies and imaging.
The second near-IR region of the electromagnetic spectrum (1000-1700 nanometers) ...
Researchers at the University of Colorado Anschutz Medical Campus have found a promising drug candidate that could help restore vision in individuals with multiple sclerosis (MS) and other neurological conditions that damage neurons.
The study was published this week in the journal Nature Communications.
The drug, LL-341070, enhances the brain's ability to repair damaged myelin— the protective sheath around nerve fibers. Damage to myelin is a hallmark of diseases like MS, as well as a natural consequence of aging, often resulting in vision loss, loss of motor skills, ...
Complex organisms, thousands of times smaller than a grain of sand, can shape massive ecosystems and influence the fate of Earth's climate, according to a new study.
Researchers from Arizona State University, along with their colleagues from the National University of the Peruvian Amazon, have identified an unknown family of microbes uniquely adapted to the waterlogged, low-oxygen conditions of tropical peatlands in Peru’s northwestern Amazonian rainforest.
The new research shows these microbes have a dual role in the carbon cycle and the potential to either ...
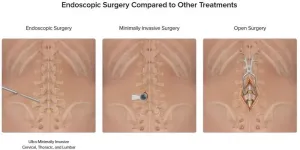
CLEVELAND – University Hospitals is now offering endoscopic spine surgery for patients needing treatment for back pain due to herniated discs in their spine. Xiaofei (Sophie) Zhou, MD, completed Arthrex's Endoscopic Spine Training course to bring this advanced procedure to the health system and recently completed the first endoscopic discectomy utilizing Arthrex technology at UH. The health system is the only one in the greater Cleveland area offering this type of ultra-minimally invasive surgery.
Arthrex's technology allows surgeons to remove the ...
Reston, VA (January 24, 2025)—The Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM) have issued a new procedure standard/practice guideline for the use of fibroblast activation protein (FAP) PET. Published in the January issue of The Journal of Nuclear Medicine, the procedure standard/practice guideline was developed to assist providers in recommending and performing FAP PET, as well as interpreting and reporting results of the imaging studies.
FAP is a transmembrane protein expressed on both cancer-associated fibroblasts and on normal activated fibroblasts involved in wound healing and ...
The National Science Foundation Convergence Accelerator Program has granted $5 million dollars to Phase 2 of the project “Securing critical material supply chains by enabling phOtovoltaic circuLARity (SOLAR).”
SOLAR’s goal is to proactively ensure circularity of solar panels by providing solutions to barriers throughout the end-to-end supply chain. The intent is to make solar panels recyclable and find a solution to remanufacturing them at a competitive cost. Achieving this will help promote a clean and resilient energy system in the United States.
The three-year project is led by Battelle Memorial Institute ...
NEW YORK, NY, January 24, 2025 — A team of scientists has developed a groundbreaking approach using specially designed peptides to improve drug formulations. This innovative method significantly enhances anti-tumor efficacy, as demonstrated in leukemia models. The study, published in the journal Chem, was led by researchers at the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC) and Memorial Sloan Kettering Cancer Center.
Drug delivery systems often face two critical challenges: poor solubility and inefficient delivery within the body. Many drugs do not dissolve well, making it difficult for them to reach ...
ST. LOUIS, MO, January 24, 2025 - A new collaborative research team of leading plant scientists are developing sorghums with nitrogen-saving traits by utilizing the genetic diversity of wild relatives to improve resilience and productivity for grain sorghum producers.
The project is part of a $38 million investment in nine projects by the U.S. Department of Energy, DOE, Advanced Research Projects Agency-Energy, ARPA-E, to develop advanced technologies for plants to increase nitrogen-use efficiency and reduce nitrogen pollution from U.S. bioenergy feedstocks.
Veena Veena, PhD, MBA, principal investigator and director of the Plant Transformation ...
Researchers from Mass General Brigham and collaborating institutions have developed a non-invasive approach to manipulate cardiac tissue activity by using light to stimulate an innovative ink incorporated into bioprinted tissue. Their goal is to develop a technique that can be used to repair the heart. Their findings in preclinical models, published in Science Advances, show the transformative potential of non-invasive therapeutic methods to control electrically active tissues.
“We showed for the first time that with this optoelectronically active ink, we can print scaffolds that allow remote control of engineered heart tissues,” said co-corresponding ...
Boston – Dana-Farber Cancer Institute researchers have identified factors that determine whether donor lymphocyte infusion (DLI), a standard therapy for patients with acute myeloid leukemia (AML) who have relapsed after allogenic hematopoietic stem cell transplant, will successfully move the patient into remission. The team identified that a key cell type in the DLI product and features of the tumor microenvironment in patients both play a role.
The findings were published in Science Immunology.
“Relapse of AML after stem cell transplant is a major challenge,” says first author Katie ...