Thioxanthones are fascinating organic compounds that have found their way into many industrial and everyday applications. In the printing industry, for example, they help inks dry faster when exposed to light thanks to their light-absorption properties, making the printing process quicker and more efficient. Some thioxanthones have been developed into FDA-approved drugs used to treat parasitic infections and cancer. Additionally, their effectiveness as photocatalysts has led some researchers to explore their potential as stabilizers against electrical breakdown. Thioxanthones have also been utilized as intermediates in molecular motor synthesis, hence novel methods for thioxanthone synthesis could accelerate the development of innovative molecular motors for nanotechnology applications.
Despite their remarkable versatility, creating functional thioxanthones can be quite challenging. This is because they have a core structure that consists of a three-ring system, with a sulfur atom in a specific position in the middle ring. Thus, their synthesis often involves multiple complex steps, requiring precise conditions to ensure the right chemical bonds without altering the functional groups.
In an effort to overcome these hurdles, a research team from Japan has developed a novel method to prepare complex thioxanthones from relatively simple and accessible precursors. Their study, led by Associate Professor Suguru Yoshida from Tokyo University of Science (TUS), was published online in Organic Letters on January 9, 2025. The team included Mayu Kawada, a second-year master's student; Shinya Tabata, who obtained a master's degree in 2023; and Yukitaka Hoshi, a first-year master's student, all from TUS.
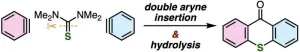
The proposed synthesis strategy involves double aryne insertion. Arynes are highly reactive molecules similar to benzene rings but have a missing pair of electrons, creating an unstable triple bond. The researchers screened various symmetrical compounds containing a double sulfur bond in the center, which would become part of the middle ring with a sulfur atom in the resulting thioxanthone molecule following aryne insertion. After testing under various reaction conditions, they identified N,N'-dimethylthiourea, as the compound that exhibited the best yields.
Leveraging the double aryne insertion strategy, the researchers were able to synthesize a wide variety of thioxanthones, including tetrasubstituted, asymmetric, multisubstituted, and even the highly challenging π-extended thioxanthones. These impressive results underscore the potential of the proposed synthesis method. “Thioxanthone skeletons are promising not only as functional molecules, such as fluorescent molecules and photocatalysts, but also as key intermediates for the preparation of various derivatives, such as thiopyrylium salts, with applications in optics, dyes, and chemical sensors,” remarks Yoshida, “Therefore, synthesizing thioxanthones via double aryne insertion would enable us to study a large variety of newly designed thioxanthone derivatives and thiopyryliums.”
It is worth noting that recent advancements in aryne synthesis techniques have made their production more accessible. This study leverages these advancements to efficiently incorporate arynes into the synthesis of thioxanthones, opening up new and exciting possibilities in organic chemistry. Considering the proposed strategy is straightforward and involves few steps, it has the potential to lower the production costs of drugs and industrial chemicals derived from multisubstituted thioxanthones, making compounds more accessible and reducing environmental impact. Moreover, the ability to easily synthesize π-extended thioxanthones opens up new avenues for developing materials with enhanced electronic and optical properties. This could lead to more efficient organic semiconductors for electronic devices and improved light-absorbing materials for solar cells.
Yoshida and his team are already working on the next steps after the favorable results they achieved in this work. “Further studies are ongoing in our research group, including the synthesis of various functionalized thioxanthone derivatives involving unsymmetric thioxanthones, detailed theoretical analysis of the proposed strategy, and the applications of thioxanthone-derived functional materials, such as thioxanthene-type molecular motors,” concludes Yoshida.
Only time will reveal the full impact of this breakthrough on the field of organic chemistry.
***
Reference
DOI: 10.1021/acs.orglett.4c04490
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan's development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society," TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today's most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Associate Professor Suguru Yoshida from Tokyo University of Science
Dr. Suguru Yoshida is an Associate Professor at the Faculty of Advanced Engineering, Department of Biological Science and Technology, Tokyo University of Science, Japan. He received his B.S. and M.S. degrees from the University of Tokyo. He earned his Ph.D. in engineering from Kyoto University in 2009 and held postdoctoral roles at Kyushu University and the University of Hawaii, before becoming Associate Professor at Tokyo Medical and Dental University and Program Officer at MEXT. A recipient of the Thieme Chemistry Journals Award and MEXT's Young Scientists' Prize, he has published over 130 articles with 3,600+ citations in synthetic organic chemistry and chemical biology.
Funding information
This work was supported by JSPS KAKENHI Grant Number JP23K23354 and Tokuyama Science Foundation.
END